 Neurobiology
Neurobiology
Visualizing the effects of sleep on neurons’ maintenance
All animals sleep, and the loss of sleep result in significant deficits to brain performance. Prolonging sleep deprivation could be fatal; however, why do we sleep is still largely an open question. Our research suggests that single neurons require sleep to maintain their DNA.
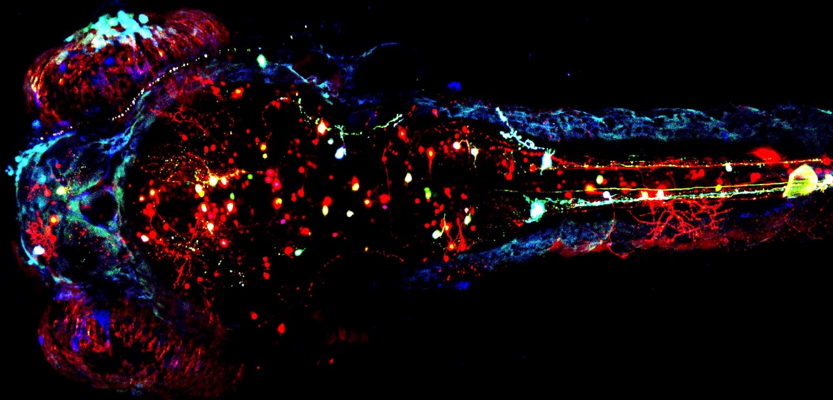
Why do we spend a third time of our lives sleeping? Why do all animals with a nervous system sleep? And how can we define sleep in all species? From jellyfish to mice to humans, all animals sleep despite the risk of predation and survival. Remarkably, the evolutionarily conserved role of sleep remains a mystery and is considered among the biggest unanswered questions in life sciences.
Much theoretical and experimental research was conducted in an attempt to explain the function of sleep. Indeed, sleep is essential for brain performance, and aids in vital processes, such as metabolic clearance, energy conservation, macromolecule synthesis, synaptic plasticity, learning, and memory consolidation. However, even animals with a basic nerve net require sleep, and sleep in local brain regions was observed in animals such as marine mammals. These observations suggest that sleep could be vital for the function of single neurons.
In our recent study, we used the tiny fish Danio rerio, also called "Zebrafish", to study the cellular mechanism of sleep in live animals. Zebrafish is a vertebrate with a conserved yet relatively simple brain compared to mammals, which sleeps during the night, as is the case in humans. Zebrafish are highly amenable for genetic manipulations, and their transparent brain gives a unique opportunity to image live neurons within the brain in the context of physiological behaviors such as arousal and sleep. We developed 3D imaging techniques to image and quantify the movements of basic DNA structures - chromosomes - within single neurons during the day, night, and sleep manipulations. Surprisingly, sleep increases chromosome movements compared to wakefulness. These sleep-dependent increased movements appeared to be unique to neurons and were not detected in two other cell types.
Why do DNA structures increase their movements during sleep? How do the single neurons, the whole brain, and the entire animal benefit from sleep-dependent increased DNA movements? Analyzing single neurons, we showed that during wakefulness, when chromosome movements are low, DNA damage increases. This accumulation in DNA damage can be the result of chemical species containing oxygen, natural nuclear processes to expedite gene expression, and even neuronal activity. The accumulation of DNA damage eventually triggers sleep that increases the movements of the DNA, which is essential for efficient repair of the DNA damage. If sleep-dependent DNA movements are inhibited in single neurons, DNA damage keeps accumulating, which may lead to malfunctioning neuronal networks and possibly cell death. This could explain how sleep disturbances affect brain performance, aging, and various brain disorders.
Altogether, this work on zebrafish suggests that the role of sleep is to enable efficient nuclear maintenance in neurons. An intriguing allegory could be a city center. During the daytime rush hours, the roads accumulate potholes, wear, and tear. Although we can fix this damage during the day, the most convenient and efficient time to maintain and repair the roads is during low traffic nighttime hours.
These findings open new research capabilities and fields in neuroscience and sleep research. It shifts cell biology experiments into a live transparent, and behaving animal. Furthermore, this work links sleep behavior with processes at the levels of single cells and molecules. It suggests that quantification of chromosome movements can be used to define sleep in individual cells and that one of the functions of sleep is nuclear maintenance. However, further experiments should be performed in new organisms, ranging from cnidarian to mammals, in order to test whether these findings are conserved across all animal kingdom. Regardless, this work provides a possible explanation for why sleep is not a luxury - it is mandatory.
Original Article:
D. Zada, I. Bronshtein, T. Lerer-Goldshtein, Y. Garini, L. Appelbaum, Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun 10, 895 (2019)Next read: When the girdle of social timing relaxes: Effects of the COVID-19 lockdown on human sleep by Christine Blume , Marlene H. Schmidt
Edited by:
Massimo Caine , Founder and Director
We thought you might like
How nanosized shrapnel from exploding fungal cells may impact us: from allergies to cloud formation
Nov 5, 2020 in Microbiology | 3.5 min read by Michael J. Lawler"Peeling back the onion": a multi-layered approach to understand the dynamics of sleep
Apr 17, 2019 in Neurobiology | 4 min read by Maxime Jan , Paul FrankenSleep or die: how good sleep decreases the risk of Alzheimer’s disease
Oct 23, 2019 in Health & Physiology | 3.5 min read by Akira OhkuboMore from Neurobiology
New, smaller-than-ever devices to help us understand how our brain works from the inside
Nov 8, 2024 in Neurobiology | 4 min read by Filippo DonatiCan we use a magnet to see brain inflammation?
Sep 25, 2023 in Neurobiology | 4 min read by Raquel Garcia-Hernandez , Santiago Canals , Silvia de SantisSurprising Behavior Changes in Genetically Modified Syrian Hamsters
Aug 30, 2023 in Neurobiology | 4 min read by Susan Lee , Kim Huhman , Jack TaylorTo achieve goals, we definitively need our neurons
Mar 10, 2023 in Neurobiology | 3.5 min read by Julien CourtinThe Impact of SARS-CoV-2 on the Brain: It Is All in Your Head
Feb 15, 2023 in Neurobiology | 3.5 min read by Meredith G. Mayer , Tracy FischerEditor's picks
Trending now
Popular topics


