What is going on with SARS-CoV-2 infection and your brain? Even in mild infection there may be neurological injury that affects recovery. In our study, brain tissue recovered from SARS-CoV-2 infected non-human primates revealed microbleeds, neuron injury and death, and evidence of brain hypoxia, all of which may cause long-lasting neurological symptoms after infection.
Views 3443
Reading time 3.5 min
published on Feb 15, 2023
Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with respiratory illness, infection can cause a variety of neurological complications. In fact, many individuals report neurological symptoms during infection in the absence of fever, cough, shortness of breath, and/or other symptoms more typical of infection. Neurological complications of infection range in severity from severe and life-threatening, such as stroke or altered mental status, to comparatively mild, including impaired smell and/or taste and intense headache. Even less severe neurological manifestations of infection can be very unpleasant and may prolong recovery for these patients.
While it’s clear that SARS-CoV-2 infection can cause major neurological symptoms, how it does this is less clear. In our recent work, we examined brains of infected non-human primates to gain insight into how infection affects the brain. Non-human primates (NHPs) are susceptible to SARS-CoV-2 infection and have brain structures and cell processes similar to humans, which make them an important animal model for advancing our understanding of brain pathology (disease) in infection. This study reported the brains of SARS-CoV-2 infected NHPs have pathology that is consistent with that seen in brain of infected human patients, including wide-spread inflammation, small bleeds into the brain, called microhemorrhages, and neuronal injury and death.
We have observed evidence of inflammation in all brain regions examined from the infected animals. This is seen as increased proteins by microglia, the resident immune cell in the brain, and astrocytes, cells that support neuron health and function. The protein Iba-1 is normally expressed by microglia but increased in expression under inflammatory conditions. Similarly, GFAP is normally expressed by most astrocytes and upregulated in the context of inflammation. Both Iba-1 and GFAP are upregulated in the brain of infected, as compared to non-infected, animals and reveal changes in microglia and astrocyte morphology (shape) that indicate inflammation.
More concerning is the increase in small bleeds in the brain, called microhemorrhages, and neuron injury and death seen in infection, as compared to non-infected animals of similar age. Microhemorrhages can result from the development of a thrombus, or blood clot, comprised of platelets and red blood cells. Our model, however, shows most bleeds in infected animals are not associated with clots, as demonstrated by a lack of CD61, a platelet derived clotting protein, in vessels associated with microhemorrhages.
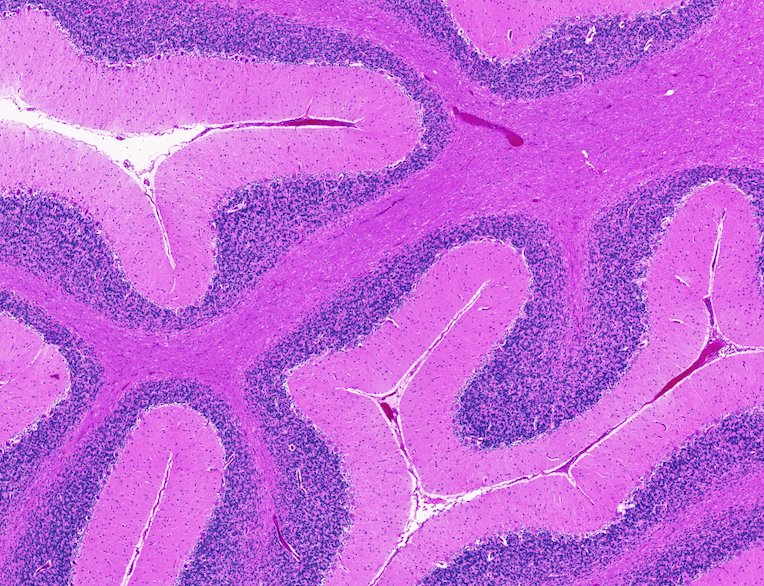
Only in infected animals we observed areas of injured and/or dying neurons, as demonstrated by cytoplasmic vacuoles, or holes, within the cells and damaged nuclei. Large neurons in the cerebellum, called Purkinje cells, are most notably affected. Positivity of cleaved caspase 3, a protein that indicates apoptosis (cell death), is only seen in brain of SARS-CoV-2 infected animals.
Microhemorrhages and neuronal injury/death are most prevalent in deep brain structures in infection. It is reasonable to suspect these bleeds contribute to neuronal injury/death, as they would cause hypoxia, or reduced oxygen, due to reduced blood flow, in the brain. We examined brain of infected and non-infection animals for hypoxia by investigating brain for the presence of hypoxia inducible factor-1a or HIF-1a. HIF-1a is a subunit of the transcription factor HIF-1 and is stabilized in response to low oxygen levels. Our study demonstrated HIF-1a is increased in infection, predominantly by cells that comprise the brain vasculature, as well as cells in the surrounding area.
What role, if any, does the virus directly play in SARS-CoV-2-associated brain injury? Interestingly, only minimal virus is seen in the brain regions we investigated, and no virus is detected in cerebrospinal fluid (CSF). This is consistent with multiple reports of infected human patients. A caveat of this study, however, is the olfactory bulb and olfactory tract tissues were unavailable for these animals and may be a possible route for viral entry into the brain. Even if this does occur, in our study virus did not spread to the deep brain structures where we see significant injury. In the limited cases where virus is observed in brain of our infected animals, it appears to localize to cells that line the blood vessels (endothelial cells).
Importantly, brain injury in SARS-CoV-2 infected NHPs is seen even in the absence of severe respiratory disease. This may provide some insight into the neurological complaints of individuals who only developed asymptomatic or mild disease. As such, it is important to evaluate patients with SARS-CoV-2 infection, even when mild, for neurological issues. Additional research investigating the cellular events that lead to the development of inflammation, microhemorrhages, and neuronal cell death in the brain is critical for preventing and/or treating neurological complications of SARS-CoV-2 infection.
Original Article:
Rutkai, I., Mayer, M. G., Hellmers, L. M., Ning, B., Huang, Z., Monjure, C. J., Coyne, C., Silvestri, R., Golden, N., Hensley, K., Chandler, K., Lehmicke, G., Bix, G. J., Maness, N. J., Russell-Lodrigue, K., Hu, T. Y., Roy, C. J., Blair, R. v., Bohm, R., … Fischer, T. (2022). Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nature Communications, 13(1), 1745. https://doi.org/10.1038/s41467-022-29440-z
 Neurobiology
Neurobiology