 Neurobiology
Neurobiology
Finding the one: what prairie voles can tell us about the drive to seek out our romantic partner
Like us, monogamous prairie voles can form lifelong pair bonds. We asked how these long-term bonds change brain activity in brain regions associated with reward and motivation. We discovered a set of cells that are active when a vole approaches their partner. The number of these cells expands as bonds mature, potentially encoding the growing desire to be with a pair bonded partner.
As humans, we fall in love and "couple-up", something scientists refer to as a pair bond. This is unusual among mammals, most of whom are promiscuous - typically mating and moving on. Fewer than 10% of mammalian species share our ability to form pair bonds, and of these, the monogamous prairie vole has become quite famous.
Mention of this small rodent from the Great Plains of North America has become frequent both in University textbooks and the popular press (especially around Valentine's day). Like many humans, prairie voles form bonds with their partner and establish a shared home, and both parents raise their young together. Also like us, individual voles will display these behaviors to different degrees: Some will be extremely engaged and exclusive in their pair bond, while others will "wander". However, what has enamored neuroscientists is not the reality-show-like drama that may play outin wild grasslands, but rather that these small rodents provide unique opportunities to study the monogamous brain in the laboratory.
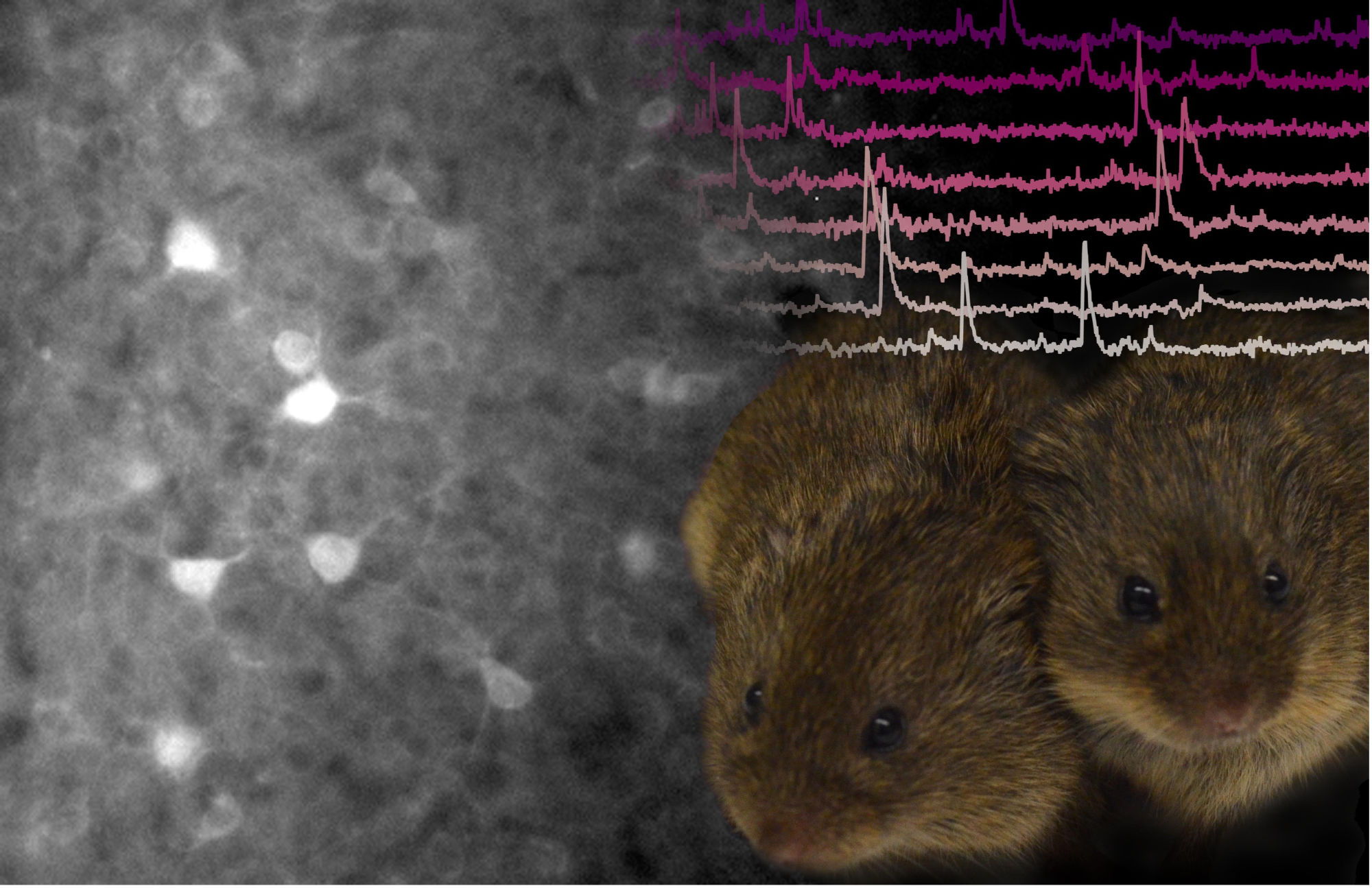
The brain is made up of billions of interconnected nerve cells that send electrical signals to each other. These signals are the basis for all the processes our nervous system performs: thinking, sensing, doing. A major goal of neuroscience is to figure out how these signals form the code for a given mental process, and as social neuroscientists, we were particularly interested in what this code might look like for something akin to love in prairie voles. Accessing these signals can be challenging, but fortunately, we were able to take advantage of new technologies that enabled us to visualize this code - turning brain signals, which are normally invisible like radio transmissions, into flashes of light. This approach cannot look at the entire brain at once, so we focused our efforts on one particular brain region: the nucleus accumbens. We chose this area of the brain because it likely combines sensory information about a partner (what do they look and smell like?) with reward and emotional information (how good do I feel right now?).
To study the nucleus accumbens, we used a benign version of a virus to deliver a new gene into the cells in this brain region, which made them glow green like a deep-sea jellyfish. The gene we used didn't just make neurons glow - it made them flicker brighter or dimmer as electrical signals were sent and received. To see all this, we attached tiny microscopes to our voles' heads, peering down on a sea of neurons like stars in a night sky. These microscopes are kind of like heavy top hats; they are a bit bulky but small enough that the animal can do all the things it normally does. And sure enough, even microscope-wearing pair bonded prairie voles still wanted to spend more time with their mates than with strangers. As a result, we could watch their behaviour while simultaneously peering through the microscope at the flurries of brain activity that looked like a light show.
Imagine the stars in the sky were trying to send a message – how would you decode that message? With our neuron "stars" now flickering, we asked whether cells in the nucleus accumbens had different codes for interacting with the vole's partner versus a vole it had never met before. When we first looked at the activity of all the cells combined, we found that all social interactions generally lit them up, but there wasn't more or less activity overall when voles interacted with a partner than with a stranger. However, we know that the brain can compute on many different scales, and sometimes individual cells within a brain region are key to its calculations. Therefore, we next looked at whether individual cells responded differently to the two types of social interactions. We found that the interaction with each individual resulted in signaling in a specific set of cells. These cells were sending their signals primarily when the vole was either moving towards or away from a specific animal. Since individual cells seemed to be specific to different partners, we wondered if this response was important to pair bonding. To get a sense of this, we looked at how these groups of cells changed over the course of a pair bond. We found that the number of cells that lit up specifically when the animal was moving towards its partner increased as their pair bonds matured over time. Correspondingly, the number that lit up for a novel vole decreased.
Like humans, individual prairie vole pair bonds differ; some voles spend almost all their time with their partner while others are less exclusive. Taking advantage of this individual variation, we found that animals with stronger bonds had a larger relative number of neurons signaling partner approach. This did not happen when we looked at the cells that lit up when the vole was moving away from either its partner or the stranger vole. We concluded that the signals sent by a specific subset of cells in the nucleus accumbens reflected the emergence and stabilization of a bond, potentially encoding the desire or decision to return to an absent pair bonded partner.
Why does this matter? As humans, we are hard-wired for social bonding, and our romantic relationships are some of the most powerful bonds we form. Losing these bonds can be devastating for our mental and even physical health. Our work provides insight into how our brains might encode these bonds, a fascinating and quintessential human behavior. Likewise, it creates a starting point for future studies into what happens in the brain when we lose these bonds and the desire to be with a partner goes unrewarded. Thus, our team is slowly paving a way towards understanding and potentially ameliorating the pain of loss, a goal that is of increasing priority as we face an unprecedented global pandemic.
Original Article:
Scribner, J. L. et al. A neuronal signature for monogamous reunion. Proc. Natl. Acad. Sci. U. S. A. 117, 11076-11084 (2020).
Next read: The Lego bricks of the brain by Toshihiko Hosoya
Edited by:
Dr. Ayala Sela , Associate Editor
We thought you might like
Attempts to Forget the Past Make It Harder to Remember the Present
Apr 8, 2016 in Psychology | 4 min read by Justin C. HulbertThe lifetime of memories
Jun 22, 2016 in Neurobiology | 3.5 min read by Thomas Stefanelli , Pablo MendezHow to print a brain - the initial steps
Nov 8, 2016 in Maths, Physics & Chemistry | 3.5 min read by Nieves CuboEvolution does not care
Jun 7, 2018 in Health & Physiology | 4 min read by Thomas Wilhelm , Holger RichlyMore from Neurobiology
New, smaller-than-ever devices to help us understand how our brain works from the inside
Nov 8, 2024 in Neurobiology | 4 min read by Filippo DonatiCan we use a magnet to see brain inflammation?
Sep 25, 2023 in Neurobiology | 4 min read by Raquel Garcia-Hernandez , Santiago Canals , Silvia de SantisSurprising Behavior Changes in Genetically Modified Syrian Hamsters
Aug 30, 2023 in Neurobiology | 4 min read by Susan Lee , Kim Huhman , Jack TaylorTo achieve goals, we definitively need our neurons
Mar 10, 2023 in Neurobiology | 3.5 min read by Julien CourtinThe Impact of SARS-CoV-2 on the Brain: It Is All in Your Head
Feb 15, 2023 in Neurobiology | 3.5 min read by Meredith G. Mayer , Tracy FischerEditor's picks
Trending now
Popular topics


