 Earth & Space
Earth & Space
Leveraging Earth to study how water formed on ancient Mars
Billions of years ago, Mars appears to have had abundant surface water. There is little, if any, direct evidence of the ancient atmosphere that helped make the surface warm enough for that to happen. Studying impact craters on Earth that are similar in structure to those on Mars may hold the key to resolving this ongoing problem.
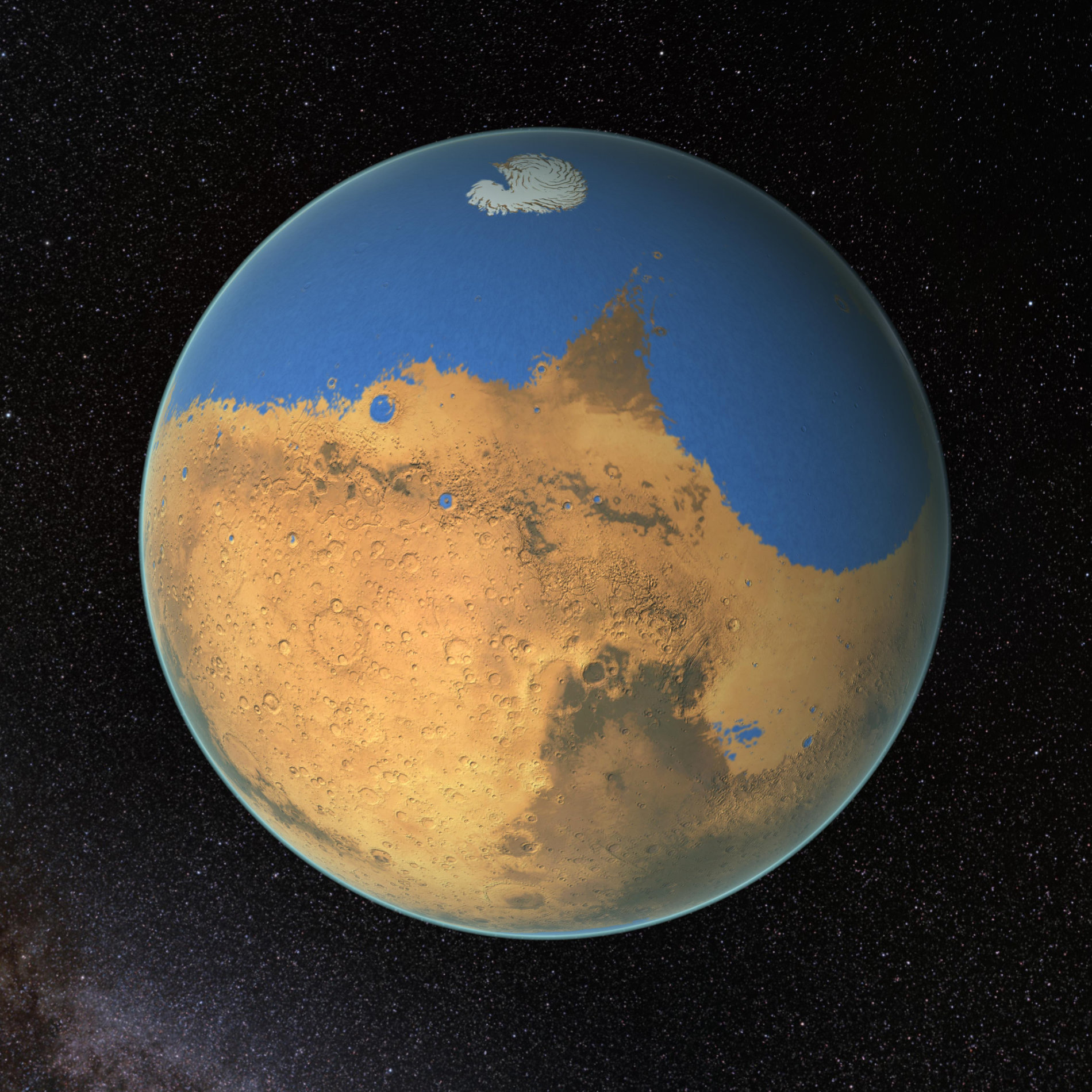
Unlike our own Blue Planet, Mars is currently too cold to sustain liquid water at its surface. At first glance, Mars 4 billion years ago should have been even colder. Back then, the Sun's brightness was only about 70% of what it is today. Yet there's a wealth of ancient channels and valley networks carved into the Red Planet's surface. Moreover, they are similar to the ones on Earth that we know are generated by flowing water. How did ancient Mars maintain liquid water despite what should have been freezing conditions? Was that water able to harbor life as we know it?
It's likely that what we presently dread on Earth was once Mars' saving grace: greenhouse gases. A lot of them – by multiplying the amount of CO2 in Earth's atmosphere today by at least 3000, you might be able to make ancient Mars' surface warm enough to support liquid water. CO2 reacts with water to generate acid, so it would stand to reason that any surface water formed during ancient Mars' lengthiest wet periods was highly acidic.
But what's been observed so far does not agree with this expectation. One possibility is that the waters were extremely alkaline – a term used to describe a solution's resistance to changes in pH (solutions with pH values under 7 are acidic, while pH values above 7 are basic). Another possibility is that ancient Mars' atmosphere was actually not consistently packed with greenhouse gases, implying that a more dynamic atmosphere was responsible for the warmth that allowed for water. The push-and-pull between these two scenarios is at the heart of a lot of ongoing research. In other words, Mars' rocks provide evidence for ancient water, but there is no consistent, corresponding set of observations for the ancient atmosphere.
When detailed visual observations cannot explain the entire picture, geochemists can analyze trends hidden within the chemistry of rocks. And while they can't go to ancient Mars, they can leverage the diversity of Earth's crust to find places that are (or were) very similar to it. Earth and Mars are both rocky planets, so they share certain surface features, like readily observable ancient craters.
We went to one such location, Ries crater in southern Germany, to explore the chemistry of rocks that formed in an ancient crater lake. The meteorite impact that formed Ries crater happened 15 million years ago, with a lake developing shortly thereafter and existing for at least 300,000 years. The impact also generated sheets of deformed rocks, called impact breccia. Ries crater has arguably the best-preserved impact breccia on Earth. Moreover, these breccias have a distinct two-layer structure that is rare on Earth but commonly observed on Mars. As water made its way into the lake, it reacted with these rocks. A previous study calculated that this increased the pH of those ancient waters to values above 9.5. To evidence this drastic pH shift, we decided to investigate the nitrogen composition at Ries Crater, as nitrogen is known to change phases as a function of pH.
As is the case with many chemical elements, there are variations in mass between individual nitrogen atoms. These are called isotopes. Variations in the nitrogen isotope contents of rocks are typically small. However, high-pH environments can lead to relatively large changes. This is because at low pH, nitrogen-bearing ammonium molecules can be stable and dissolved in water. But as pH increases, ammonium is converted to ammonia, which is more comfortable being a gas. At Ries crater, when pH increased, and ammonia gas left the waters of the lake, it tended to take lighter nitrogen isotopes with it. This also meant that heavier nitrogen isotopes tended to be left behind in the lake. That remaining nitrogen left a distinct signal in the rocks that we could clearly observe in the laboratory.
The confirmation of our hypothesis may provide useful information for the Mars 2020 (Perseverance Rover) mission, which is set to collect and return samples from Mars' Jezero crater. Like Ries crater, this location once housed a lake and was surrounded by impact breccias. Jezero's ancient lake therefore had the ingredients for a high-pH system. If it was, we would expect the high-pH nitrogen isotope signal in ancient Martian samples. However, if CO2 levels on ancient Mars were indeed thousands of times greater than the modern Earth, such a high-pH lake would have been extremely unlikely to form. Therefore, we suggest that if the distinct nitrogen isotope trend we observed in Ries Crater is absent in the samples returned from Mars, then there may have been enough CO2 in the atmosphere to acidify Jezero's lake waters.
This study provides a novel blueprint for evaluating the pH of Mars' ancient waters, which, in turn, would provide insight into its elusive ancient atmosphere. Untangling the puzzling nature of these features will be key for any valuable appraisal of if, or how, there may have ever been life on Mars.
Original Article:
Stüeken, E. E., Tino, C., Arp, G., Jung, D. & Lyons, T. W. Nitrogen isotope ratios trace high-pH conditions in a terrestrial Mars analog site. Sci. Adv. 6, 1-9 (2020).
Next read: Diving into the icy origins of Martian valleys by Anna Grau Galofre , Mark A. Jellinek , Gordon R. Osinski
Edited by:
Dr. Akira Ohkubo , Associate Editor
We thought you might like
Nitrogen pollution from lowlands reaches distant mountain lakes
Sep 21, 2016 in Earth & Space | 3 min read by Beth Hundey , Katrina Moser , Fred LongstaffeCometary nitrogenous salts tell about the Solar System’s history
Jan 15, 2021 in Earth & Space | 3.5 min read by Olivier PochNitrogen is becoming less available in ecosystems around the world
Jul 28, 2023 in Earth & Space | 3 min read by Rachel MasonMore from Earth & Space
Discovery of the first radiation belt beyond the Solar System
Jan 27, 2025 in Earth & Space | 3.5 min read by Juan Bautista Climent OliverOne million (paper) satellites
Jan 24, 2025 in Earth & Space | 3 min read by Ewan Wright , Andrew FalleVolcanic Ash: A Nutrient Boost for Reef-Building Corals
Sep 18, 2024 in Earth & Space | 4 min read by Frank Förster , Tom SheldrakeAmmonia Energy: A Call for Environmental Awareness
Aug 29, 2024 in Earth & Space | 3.5 min read by Matteo Bertagni , Robert Socolow , Amilcare PorporatoLikely increase in coral thermal tolerance at a Pacific archipelago
Dec 29, 2023 in Earth & Space | 3 min read by Liam LachsEditor's picks
Trending now
Popular topics


