 Microbiology
Microbiology
Unlocking a new way to fight against antibiotic resistance: viruses are the key
We discovered viruses, called phages, that are capable of killing an antibiotic-resistant superbug lurking in intensive care units (ICUs). When the superbug tried to escape from phages it lost the protective layer that made it antibiotic-resistant. We forced the super-bug into the dilemma of death by phages or death by antibiotics.
Most of us have suffered a bacterial infection at some point in our lives. Fortunately, a prompt prescription of the right antibiotic from our doctors puts us on the path to recovery. But in re-cent years, for an increasing number of patients, antibiotic treatments are failing. This is espe-cially true if the place where the infections are acquired is a hospital.
Think about the amount of disinfectants, antibiotics, and cleaning procedures that are used in hospitals. Sure enough, they kill most of the microbes living there. But the ones that survive are ‘superbugs’, that is, they are bacteria that have evolved to resist all of those threats. Su-perbugs prey on the already vulnerable hospitalized patients, who frequently have underlying conditions and weakened immune systems. It’s the perfect storm.
The specific superbug our research tries to fight is Acinetobacter baumannii (or A. baumannii, for short). It is responsible for up to 20% of infections in ICUs, affecting the lungs, wounds, and even bloodstreams, and carries a high mortality rate. In recent years, A. baumannii has be-
come incredibly resistant to antibiotics, prompting the World Health Organization to call it a ‘critical threat’.
Let us take a detour here and recognize how the COVID-19 pandemic, caused by a virus, has turned our lives upside down. It might lead you to think all viruses are dangerous, and hardly anyone could blame you. However, referring back to our novel research, you might be sur-prised to know that we work with viruses that can be our allies.
Bacteriophages (or phages) are viruses that—instead of infecting and killing human cells—only infect and kill bacteria. Phages are everywhere, from the soil outside our houses, to oceans, to even our guts and skin. They are quite picky: a certain phage will only kill a specific type of bacteria. This is because the proteins on the tail end of a phage need to match and attach to receptors on the surface of the bacterial cell in a lock-and-key fashion to initiate infection. Our project began hunting for phages that had the key to kill A. baumannii.
We found the phages we were looking for in raw sewage (untreated waste water) which is in-credibly rich in all sorts of microbes. We cleaned and characterized them. When we added them to cultures of A. baumannii, we were pleased to see they annihilated the bacterial popu-lation, or so we thought.
Through the process of evolution, many bacteria can outsmart life threatening agents, includ-ing antibiotics and phages. In our experiments, after merely 8 hours we observed the emer-gence of a bacterial population capable of resisting the phages’ attack. They were phage-resistant mutants.
Our research took a turn, and we sought to discover the method through which A. baumannii had become phage-resistant. To do this, we first compared the genetic makeups of the origi-nal bacteria and the phage-resistant mutants to find the differences between them. We found that the phage-resistant mutants had errors in the genes that would normally contain the in-structions to produce a bacterial structure called ‘capsule’.
The bacterial capsule is an outer layer made up of sugars. It covers and protects bacteria; because it is sticky, it helps bacteria attach to medical devices and human tissues; and be-cause it is thick and viscous, it stops the entry of antibiotics.
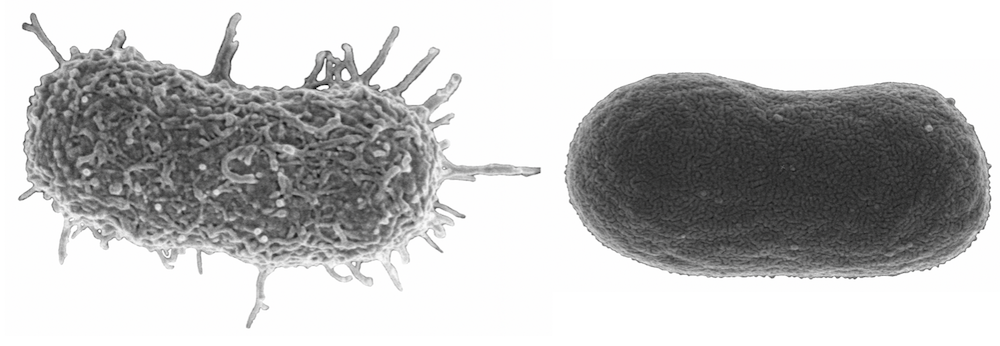
Our genetic analysis suggested that the phage-resistant population lacked these capsules, however, we looked for further confirmation. We used high power microscopy to explore the surface of the bacteria. The picture atop this article shows our results. To the left, the original A. baumannii has its beautiful sticky capsule intact; to the right, the surface of phage-resistant A. baumannii looks flat and dull. The capsule is gone.
Next, we questioned why the lack of capsule was protecting A. baumannii from our phages. Utilizing an ‘adsorption assay’, where we test if phages can bind to bacteria, we discovered that our phages could not perform the lock-and-key recognition of capsule-deficient bacteria. Our phages had the key, but the lock was nowhere to be found.
However, because we knew that capsules prevent the entry of antibiotics, we tested if A. baumannii had lost some of its resistance to antibiotics. Out of nine tested antibiotics, we ob-served this ‘resensitization’ effect with seven of them. In trying to escape from phages, A. baumannii had let its guard down against antibiotics.
Our results show that we can take advantage of bacterial evolution and use phages and anti-biotics in combination to treat this dangerous superbug. We are currently working on animal trials of our therapeutic one-two-punch approach. Our end goal is to have a proven weapon against A. baumannii infections and, hopefully, to make ICUs safer.
Original Article:
https://doi.org/10.1038/s41564-020-00830-7Next read: How much can antibiotic prescription rates be reduced through targeted interventions? by Kyaw Zay Ya , Mark Lambiris , Günther Fink
Edited by:
Dr. Devina Misra , Senior Scientific Editor
We thought you might like
More from Microbiology
Monoclonal antibodies that are effective against all COVID-19 -related viruses
Jan 31, 2024 in Microbiology | 3.5 min read by Wan Ni ChiaPlagued for millennia: The complex transmission and ecology of prehistoric Yersinia pestis
Jul 31, 2023 in Microbiology | 3 min read by Aida Andrades Valtueña , Gunnar U. Neumann , Alexander HerbigHow cellular transport can be explained with a flip book
Jun 5, 2023 in Microbiology | 3 min read by Christina ElsnerThe Achilles’ heel of superbugs that survive salty dry conditions
Apr 24, 2023 in Microbiology | 4 min read by Heng Keat TamNew chemistry in unusual bacteria displays drug-like activity
Mar 21, 2023 in Microbiology | 3.5 min read by Grace Dekoker , Joshua BlodgettEditor's picks
Trending now
Popular topics


