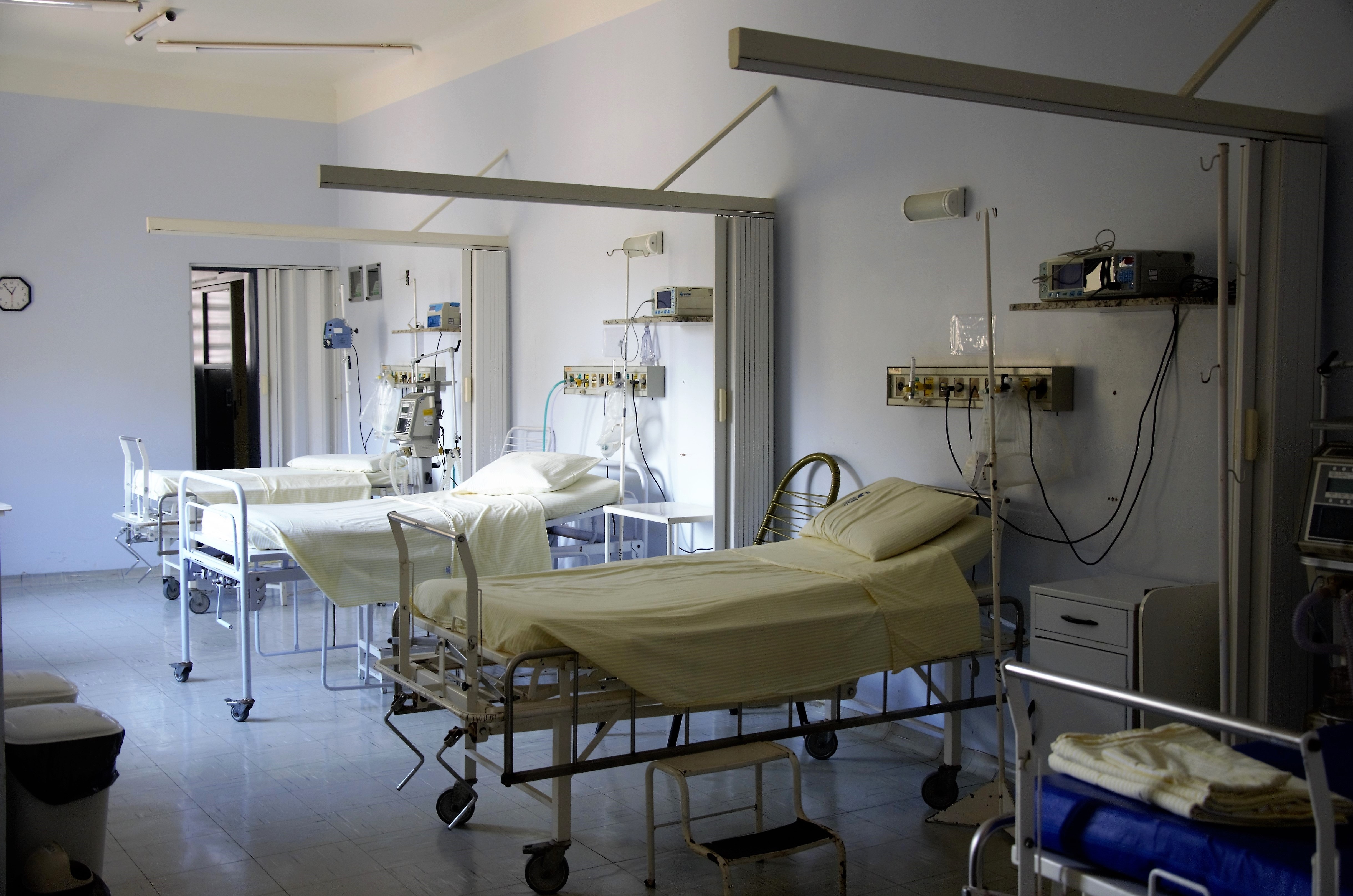
Nosocomial infection has become a serious global challenge in modern healthcare facilities due to the persistence of superbugs. To survive in highly sanitized hospitals, superbugs produce beneficial substances such as mannitol to sustain their growth. We uncovered that a mannitol-producing protein is vital to help the bugs persist in salty and dry conditions, and may be the key to their demise.
Views 4168
Reading time 4 min
published on Apr 24, 2023
Nosocomial infection is an infectious disease acquired from hospitals or other healthcare facilities. It is also known as hospital-acquired infection or healthcare associated infection. These infections are normally spread to the immunocompromised patients, particularly those who are admitted to the intensive care unit, causing severe pneumonia, bloodstream infection, and urinary tract infection, associated with high rates of morbidity and mortality. The extended use of antibiotics arising from human activities has jeopardized the effectiveness of such antibiotics in human health, contributing to the development and dissemination of MultiDrug Resistant (MDR) bacteria, and an increased economic burden due to higher medical costs and prolonged hospitalization.
The opportunistic human pathogen Acinetobacter baumannii is one major cause of nosocomial infections and this bacterium accounts for ~20% of the infections associated with the intensive care unit globally. In 2017, it was listed by the World Health Organization as the most critical bacterium in search of new antibiotics, owing to its MDR phenotype. One key aspect of the persistence of A. baumannii in hospitals is the long-term survival in harsh environments such as extreme dryness and high salinity. Indeed, this pathogen can live on the hospital surfaces for weeks if not months, if the surfaces are not disinfected properly. To survive in these conditions, this deadliest bacterium employs several strategies to mitigate dehydration. Often it produces substances that protect itself against drought and salt, or by taking up those substances from the environments. These substances are so-called compatible solutes, which are used to retain water, thereby preventing dehydration. One of the main compatible solutes made by this bacterium is a sugar alcohol known as mannitol and it is better known as low calorie sweetener.
In some organisms, the production of mannitol features two consecutive processes involving two separate protein modules. The first process in mannitol synthesis involves the conversion of a fructose derivative, fructose-6-phosphate to mannitol-1-phosphate, carried out by the first protein module, the mannitol-1-phosphate dehydrogenase (M1PDH) module. Fructose is a sugar mainly found in honey and berries. Subsequently, mannitol-1-phosphate is converted to mannitol by the second protein module, the mannitol-1-phosphate phosphatase (M1PP) module. Our group was struck by several features of this pathogen. A. baumannii has evolved an unusual mannitol-producing protein (AbMt1D) by joining both M1PDH and M1PP modules, to form a M1PDH/M1PP-linked unit by the evolutionary process. It is one of only three bacteria known to carry such a unique M1PDH/M1PP-linked unit.
Since our colleagues discovered the decisive role of mannitol in the survival of A. baumannii on dry surface and high salinity, there is a pressing need to explore in depth the underlying molecular mechanism of mannitol biosynthesis in this bacterium. To understand how those AbMtlD proteins protect the bacterium in these environments, we produced AbMtlD in the Escherichia coli bacterium, the causative pathogen of traveller's diarrhoea, as the workhorse of protein production. AbMltD protein was extracted from E. coli and microscopic protein crystals were grown from pure AbMtlD protein. The journey to obtain protein crystals is time-consuming and tedious, and often leads to failure. In fact, we have carried out numerous trials and errors and, successfully produced the AbMtlD protein crystals. In collaboration with scientists from the SOLEIL Synchrotron radiation facility, we could visualize, on the atomic scale, the 3D-structure of AbMtlD protein by analysis of the X-ray diffraction pattern obtained from its crystal form.
The pure AbMtlD protein adopts a single M1PDH/M1PP-linked unit. To our surprise, further analysis demonstrated that increased salinity triggers clustering of two individual M1PDH/M1PP-linked units to form a sophisticated M1PDH/M1PP-M1PDH/M1PP-linked system. As such, this system improves the production efficiency and speed for manufacturing mannitol, compared to that without the formation of this complex system. Surprisingly, damage to the M1PP module within the M1PDH/M1PP-linked unit kills the bacterium. A subsequent investigation uncovered that such damage severely stops mannitol production and leads to an increased level of mannitol-1-phosphate within the bacterium. We found that this substance is highly toxic to the bacterium.
Therefore, we concluded that the evolution of the AbMt1D protein ensures a quick response to drought and high salinity, as well as to impede an accumulation of the toxic mannitol-1-phosphate within itself. Our study opens a new page in developing a novel sanitizing strategy or treatments against A. baumannii infections by specifically halting the production process of mannitol by the M1PP module with antimicrobials. With these antimicrobials as surface disinfectant, we can force the bacterium to commit suicide by producing toxic mannitol-1-phosphate, thus averting the spread of hospital-acquired Acinetobacter infections in the intensive care unit.
Original Article:
Tam, H.-K., König, P., Himpich, S., Ngu, N. D., Abele, R., Müller, V., & Pos, K. M. (2022). Unidirectional mannitol synthesis of Acinetobacter baumannii MtlD is facilitated by the helix–loop–helix-mediated dimer formation. Proceedings of the National Academy of Sciences, 119(14). https://doi.org/10.1073/pnas.2107994119
 Microbiology
Microbiology