 Microbiology
Microbiology
Killing C. difficile with targeted strikes
C. difficile poses a huge threat to human health, but the friendly bacteria of the gut provide us with a natural resistance to infection. By reprogramming naturally occurring nano-weapons, we can specifically target C. difficile without hurting these friendly bacteria. However, C. difficile can become resistant to these weapons, but do these resistant mutants pose a threat to our health?
Clostridium difficile is a bacterium that causes hundreds of thousands of antibiotic-associated diarrhoea cases every year, and these infections often prove fatal. Usually, C. difficile is unable to cause disease as the bacterium is kept in check by the friendly bacteria in the gut; the microbiota. However, antibiotic use, while often necessary, damages the microbiota and leaves C. difficile free to reproduce, release toxins, and cause disease.
C. difficile produces super hardy spores that are resistant to a myriad of stresses including high temperature, antibiotics, and even the chemicals in many cleaning products. These tough spores are also how the bacterium spreads from person to person, posing a real problem for health care professionals trying to limit its spread. This is also a problem when it comes to treating C. difficile infections. These infections are normally treated using additional antibiotics, however these also damage the microbiota further. As spores are unaffected by antibiotics, when a patient completes a course of medication they are susceptible to a relapse of infection when the spores that survive in the gut start to germinate. This highlights the need for more specific C. difficile treatments that don't harm the microbiota. This would allow time for the friendly bacteria to replenish, restoring natural resistance to C. difficile.
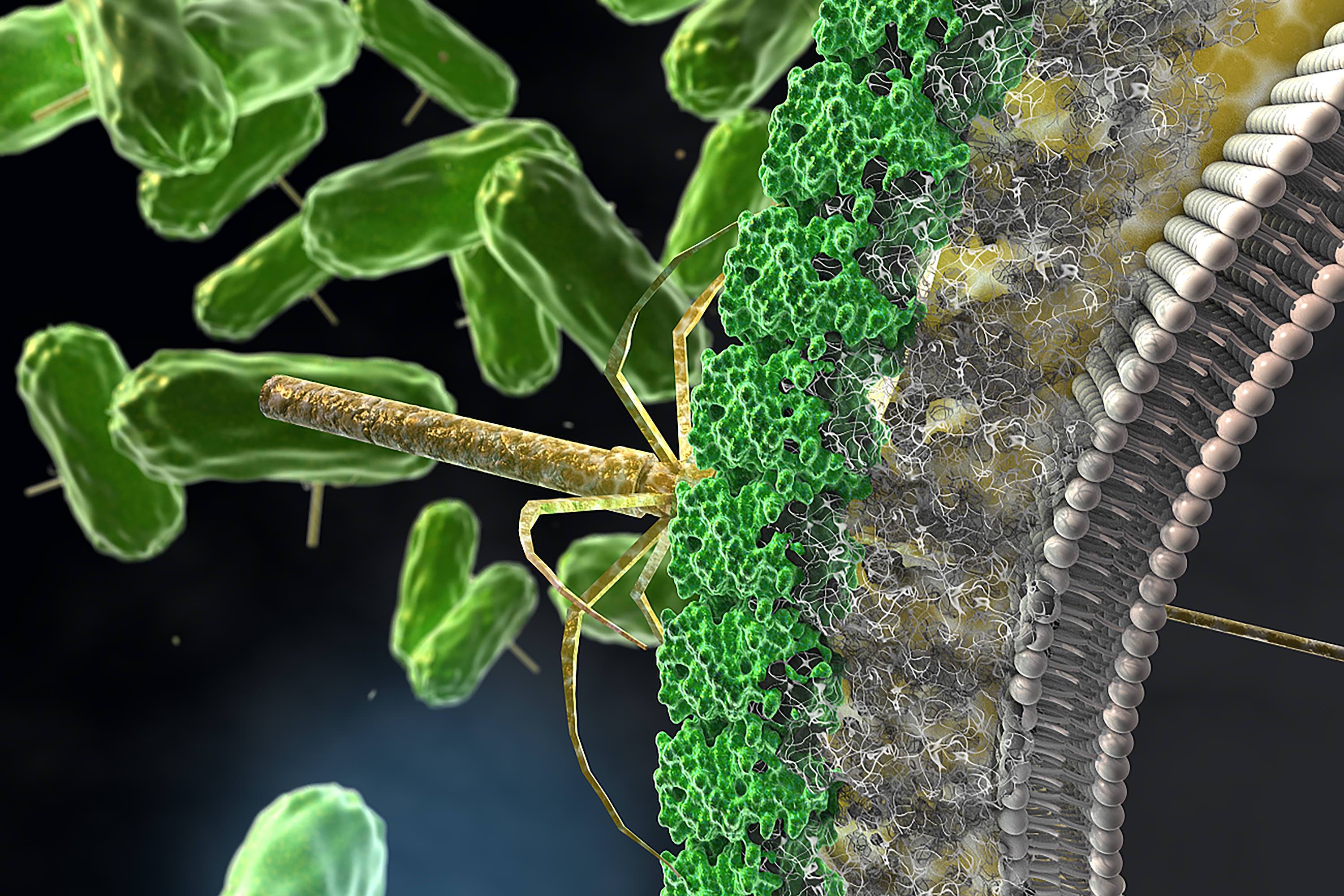
Recently, it was discovered that C. difficile makes nano killing-machines that kill competing strains of C. difficile. It seems C. difficile has a bad case of sibling rivalry. These nano-machines, called Diffocins, are shaped like oil rigs. Six legs support a body that houses a hollow drill. Upon contact with a bacterial cell, the hollow drill is pushed through the cell wall forming a hole in the bacterium, through which the bacterium's insides leak out, leading to rapid death.
So why not use C. difficile's own weapon against it?
At the foot of the Diffocins' legs there are receptor binding proteins that recognise the surface of the bacterial cell. We discovered that if we modify these proteins we can alter the strains of C. difficile that the Diffocins target. These reprogrammed weapons were named Avidocin-CDs. We produced Avidocin-CDs that target all the major disease-causing strains of C. difficile. However, there was a problem. When we exposed C. difficile to Avidocins, we isolated strains that were completely resistant to killing. If C. difficile could become resistant to Avidocins, were they going to be useful as medicines?
We decided to probe further and look at the cell surface of the Avidocin-resistant C. difficile mutants, to see how they had become resistant. To our surprise, the mutants had lost their S-layer: a protein coat that completely encases the bacterial cell in a crystal armour. S-layers are common features of bacterial physiology although very little is known about them. We sequenced the genomes of these mutants and found a mutation in the gene encoding the major S-layer protein, essentially turning it off. This gave us an exciting opportunity to answer two questions. What does the S-layer do, and are mutants without S-layers a threat to our health?
We found that these mutants grew poorly and formed strange, short, bent cells, and also showed a significant decrease in their ability to produce spores. More importantly however, we showed that these mutants could not produce toxins and showed increased sensitivity to two components of the human immune system, lysozyme and LL37.
This tells us that Avidocin-CDs target the C. difficile S-layer and that resistance can be conferred though loss of the S-layer. However, the resistant mutants pose little threat to human health as they are incapable of producing the toxins. C. difficile's toxins cause the symptoms of disease, so no toxins, no symptoms. Avidocin-CDs force C. difficile to make a difficult choice; lose the ability to cause disease or die. It is also likely that the mutants will be less able to pass from person to person as they produce fewer spores. Studying these mutants has also taught us a lot more about the roles of the S-layer, showing it is involved in cell shape maintenance, sporulation, and protection from the immune system. The S-layer is therefore a great target for the development of anti-C. difficile treatments.
Avidocin-CDs show great potential as anti-C. difficile therapeutics. Importantly, the S-layer of C. difficile is unique so these nano-machines will kill C. difficile without damaging the protective microbiota, lowering the risk of recurrent infection.
Original Article:
J. A. Kirk et al., New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Sci Transl Med 9, (2017)Next read: Vaccine hope against a sexually transmitted disease: the answer to the burgeoning rise in a superbug by Helen Petousis-Harris
Edited by:
Massimo Caine , Founder and Director
We thought you might like
Fighting back antibiotic resistance: a new hope from the soil
Feb 24, 2016 in Microbiology | 4 min read by Dan KramerCollateral damage: antibiotics disrupt the balance in the gut
Jun 2, 2016 in Microbiology | 3.5 min read by Katri KorpelaInvisible allies for healthy juvenile growth
Oct 12, 2016 in Microbiology | 4 min read by Martin SchwarzerRed in Tooth and Claw: another weapon against antibiotic resistance
Oct 3, 2017 in Microbiology | 3.5 min read by Nicholas A. IsleyMore from Microbiology
Monoclonal antibodies that are effective against all COVID-19 -related viruses
Jan 31, 2024 in Microbiology | 3.5 min read by Wan Ni ChiaPlagued for millennia: The complex transmission and ecology of prehistoric Yersinia pestis
Jul 31, 2023 in Microbiology | 3 min read by Aida Andrades Valtueña , Gunnar U. Neumann , Alexander HerbigHow cellular transport can be explained with a flip book
Jun 5, 2023 in Microbiology | 3 min read by Christina ElsnerThe Achilles’ heel of superbugs that survive salty dry conditions
Apr 24, 2023 in Microbiology | 4 min read by Heng Keat TamNew chemistry in unusual bacteria displays drug-like activity
Mar 21, 2023 in Microbiology | 3.5 min read by Grace Dekoker , Joshua BlodgettEditor's picks
Trending now
Popular topics


