 Microbiology
Microbiology
Sleeping bacteria survive antibiotic treatment and hijack the host immune system
When we take antibiotics during a bacterial infection, most of the invading bacteria will be killed. This eases the pressure on our immune system, allowing it to clean up the few live bacteria that remain. Sometimes bacteria succeed in escaping this treatment. We are one step closer to understanding how.
Since the 1940s, it has become easier to treat bacterial infections due to the discovery of antibiotics. These drugs work by corrupting active processes in bacteria, such as the ability to make DNA or proteins. By taking antibiotics when we are infected, we kill most of the invading bacteria. This eases the pressure on our immune system, allowing it to clean up the few live bacteria that remain.
Even though antibiotic treatment is often effective in limiting bacterial diseases, many types of bacterial infections often recur, even after antibiotic treatment. Common examples are recurrent urinary tract infections, ear infections, or most famously, tuberculosis. This recurrence is thought to be caused by the reawakening of "sleeping" bacteria, called persisters. Persisters are a small subpopulation of bacteria that, while normally sensitive to antibiotics, somehow manage to withstand killing by these drugs by entering into a transient antibiotic-tolerant state (i.e. the "persister state").
The persister state has usually been thought of as equivalent to a "sleeping" state for bacteria. That is, bacteria are thought to temporarily shut down their active processes that are often targets for antibiotics. So when bacteria enter into a persister state and shut down these processes, the antibiotics cannot work. Once treatment is completed, these persisters could reawaken and start a new infection episode.
With this in mind, understanding the persister state of bacteria might help us design new treatment strategies to completely eradicate an infection, including persisters.
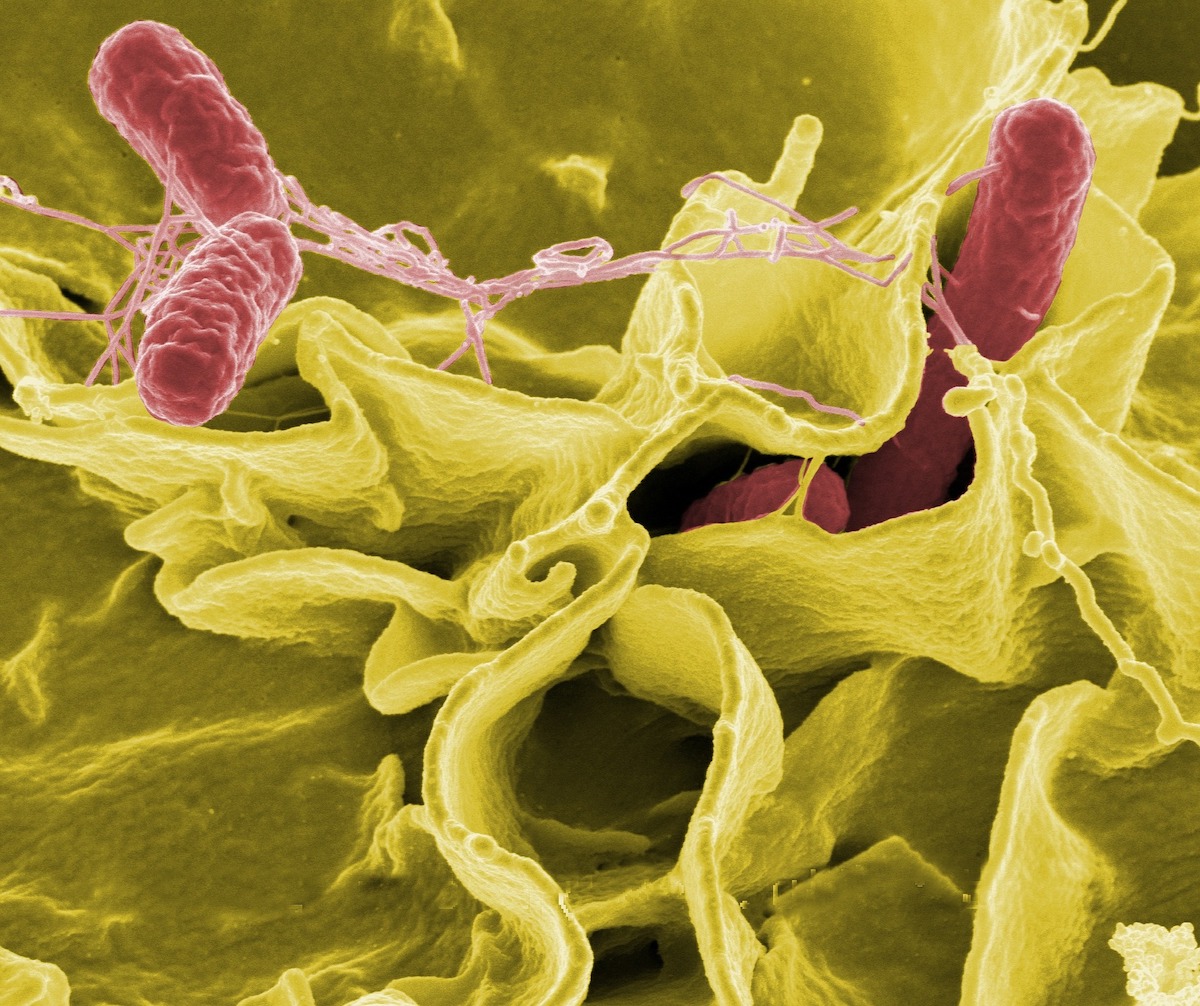
To better understand the persister state, we used Salmonella as a model bacterium. Salmonella is best known for causing food poisoning (during infection of the gastrointestinal tract) and typhoid fever (during systemic infection). The primary niche of Salmonella during systemic infection is within macrophages - immune cells designed to clear bacteria and viruses. When Salmonella is taken up by these macrophages, many of the bacteria react by entering into the antibiotic-tolerant persister state. While antibiotics cannot kill these persisters, one might assume that at least the macrophage, specialised in the killing of bacteria, could cope with these supposedly "sleeping" persister bacteria?
Surprisingly, the answer is no. Somehow, someway, these supposedly "sleeping" persisters survive. But how?
We hypothesised that possibly these so-called "sleeping" persisters were not actually sleeping. What if, quietly, persisters were manipulating our immune cells to avoid being destroyed instead?
To test this, we analysed the active processes in both the persister bacteria and in the immune cells. In every living cell, a signature for all active processes going on at that time is represented by the type and amount of specific RNA molecules. We read this RNA signature from both the host immune cells and bacterial persister cells by a technique called RNA-sequencing. Our analysis showed us that macrophages that harbour the persister bacteria initially activate processes aimed at destroying the persisters.
So why are the persisters not killed?
Our analysis also showed that rather than "sleeping", the antibiotic-tolerant persisters are in fact very much active. Specifically, in response to the macrophages trying to kill them, the bacteria strike back, synthesizing and releasing small proteins directly inside the macrophages. These small proteins function to weaken the macrophage immune response, considerably dampening its bacteria-killing capabilities.
This is quite different from the persisters that had been discovered in 1944, surviving in a laboratory culture medium, which are thought to be in a dormant "winter sleep" state, and thus completely inactive. Our results now show that persister bacteria are not completely dormant in the human body. Rather, they weaken the defence mounted by our immune cells. This means that when antibiotic treatment is completed, these persisters might have created a much more favourable environment for their own relapse, or even for infection by other bacteria or viruses.
Despite this horrible thought, it also opens up avenues to eradicate persisters. Since persisters that do not manipulate macrophages will be killed by these immune cells, inhibiting this activity of persisters, in theory, would equip our immune cells to efficiently clear hard-to-treat infections once and for all.
Original Article:
D. A. C. Stapels et al., Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 362, 1156-1160 (2018)Next read: Symbiogenesis: how algae and bacteria shaped new genes together by Raphaël Méheust , Eric Bapteste
Edited by:
Massimo Caine , Founder and Director
We thought you might like
How humans gave acne to the grapevine
Feb 28, 2015 in Evolution & Behaviour | 3.5 min read by Carlos J. Rivera-RiveraTara Oceans Expedition sequences the ocean
Nov 24, 2015 in Earth & Space | 4 min read by Chris BowlerLiving without mitochondria: the downfall of one textbook truth
Oct 3, 2016 in Evolution & Behaviour | 3.5 min read by Lukáš NovákAmoebas trap bacteria using nets of DNA: the same mechanism as human immune cells
Jan 27, 2017 in Evolution & Behaviour | 3.5 min read by Lukáš NovákMore from Microbiology
Monoclonal antibodies that are effective against all COVID-19 -related viruses
Jan 31, 2024 in Microbiology | 3.5 min read by Wan Ni ChiaPlagued for millennia: The complex transmission and ecology of prehistoric Yersinia pestis
Jul 31, 2023 in Microbiology | 3 min read by Aida Andrades Valtueña , Gunnar U. Neumann , Alexander HerbigHow cellular transport can be explained with a flip book
Jun 5, 2023 in Microbiology | 3 min read by Christina ElsnerThe Achilles’ heel of superbugs that survive salty dry conditions
Apr 24, 2023 in Microbiology | 4 min read by Heng Keat TamNew chemistry in unusual bacteria displays drug-like activity
Mar 21, 2023 in Microbiology | 3.5 min read by Grace Dekoker , Joshua BlodgettEditor's picks
Trending now
Popular topics


