 Maths, Physics & Chemistry
Maths, Physics & Chemistry
The World’s Longest Nanoscale Chain
Polycatenanes are tiny chains, made by linking cyclic molecules together in a chain without using chemical bonds. They have been attracting attention as a next-generation polymeric material. We created the world’s longest polycatenane chain using a simple solvent mixing method.
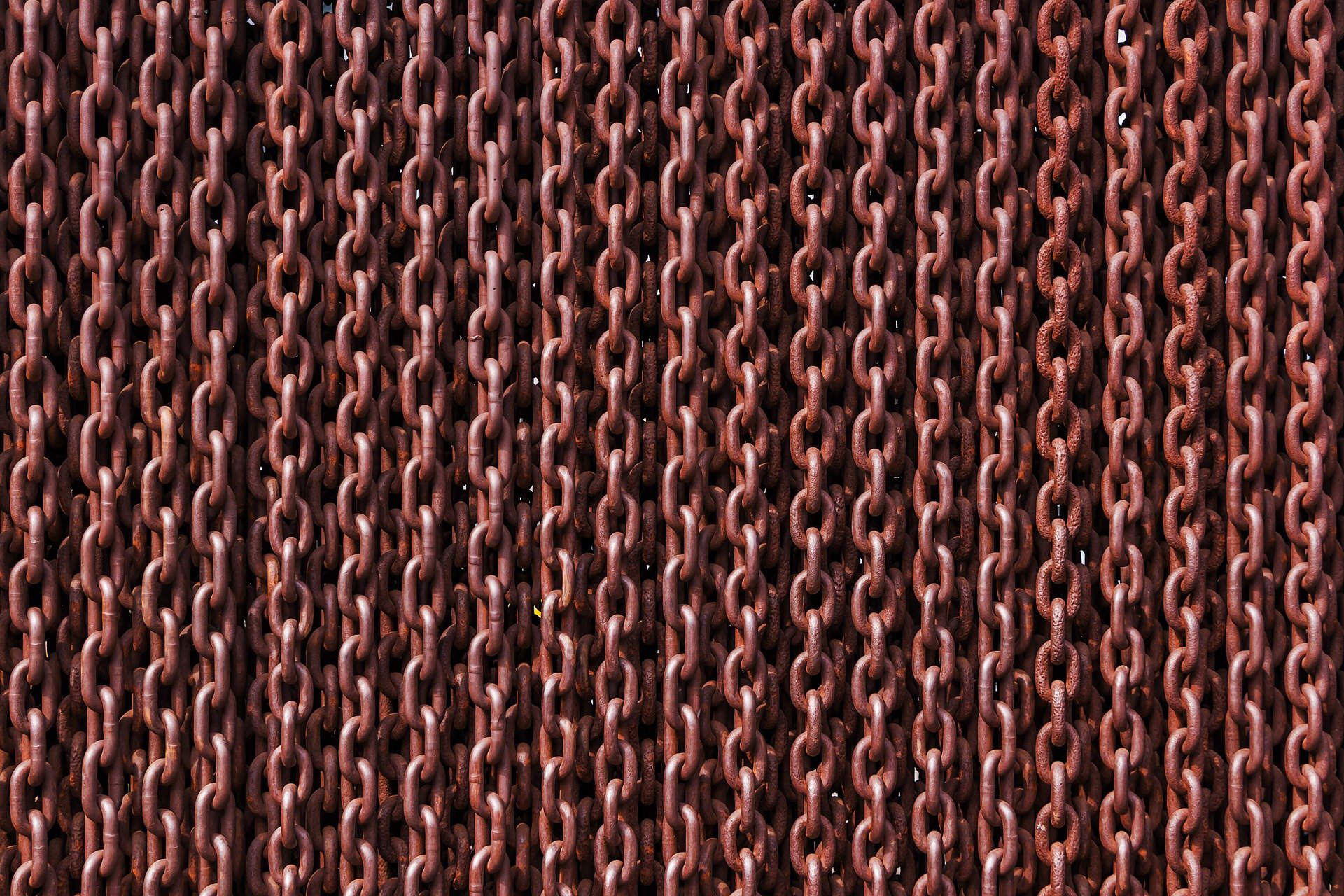
A sheet of metal or wood is an inflexible one-dimensional material. Creating flexible one-dimensional structures from such hard materials is difficult, but can be done using chain-like structures made of interlocking of rings. Moreover, chain-like structures endow materials with ease of repairing any damage by replacing only the damaged ring. Therefore, catenanes, which are chain-like structures of small ring-like molecules, have attracted great attention of chemists in many fields for their potential use.
Prof. Jean-Pierre Sauvage who is an awardee of the Nobel Prize in Chemistry in 2016, established an elegant method for the synthesis of catenanes, known as template directed synthesis. This method has been used to prepare a diverse range of mechanically interlocked molecules, including polycatenanes - catenanes composed of large numbers (up to about 30) of interlocked rings. While these polycatenanes have many potential applications as functional nanoscale materials, their synthesis is very complicated.
In our new study, we have succeeded to establish a new recipe to create polycatenane based on molecular self-assembly. While the standard synthesis method is based on making new covalent bonds (the sharing of electrons between atoms), molecular self-assembly uses non-covalent bonds. Non-covalent bonds rely on weaker interactions between distinct molecules. When many non-covalent bonds are created, they can form a strong interaction. In our case, the molecule we used as the basic subunit can form non-covalent bonds in an organic solvent. This results in interactions between six copies of it, which assemble into a structure we call rosette. The formation of rosettes then triggers another step of self-assembly, in which the rosettes become nanoscale rings.
The next step in forming catenanes is linking the rings to form chains. We achieved spontaneous catenane formation of rings by a very simple method: injecting a solution of our starting molecule in a good solvent into poor solvents. Using this simple method we achieves over 2% yield catenanes, a surprisingly high yield!
How can we explain the high yield of catenanes? We hypothesized that the formation of rings was more likely to occur on the surface of the pre-formed rings. This phenomenon - called secondary nucleation - is well known in theaggregation of misfolded proteins into amyloid fibers, wherein aggregated misfolded proteins can catalyze a change of other proteins they come into contact with. To confirm this hypothesis, we conducted a number of experiments, including a computational approach, and all of the obtained results confirmed the occurrence of the secondary nucleation on the ring surface.
Using the solvent mixing method, with different solvents allowed us to identify the conditions in which longer polycatenanes chain will spontaneously form. Finally, we observed polycatenane consisting of five interlocked rings, and we named it "Nanolympiadane" to pay tribute to Prof. Stoddart's "Olympiadane". In addition, by gradually injecting the molecule-in-good-solvent, we eventually produced polycatenane with 20 linearly interlocked rings. The length of this polycatenane is over 500 nanometers, and it is the world's longest polycatenane reported so far.
This is the first example of synthesis of a complex structure of such length through molecular self-assembly. This is a revolutionary achievement because synthesis of polycatenanes could be realized by simple mixing of molecular solutions with another organic solvent. In the future, we aim to investigate the peculiar physical and photophysical properties of the catenane chains.
Original Article:
Datta, S. et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 583, 400-405 (2020).
Next read: Reversible Anticoagulants: Inspired by Nature, Designed for Safety by Millicent Dockerill , Nicolas Winssinger
Edited by:
Dr. Ayala Sela , Associate Editor
We thought you might like
Stacking molecular chips in multiple dimensions
Aug 30, 2024 in Maths, Physics & Chemistry | 3 min read by Lucía Gallego , Romain Jamagne , Michel RickhausHow an artificial molecular machine pumps in nanoscale
May 20, 2021 in Maths, Physics & Chemistry | 3.5 min read by Quentin LaurentMore from Maths, Physics & Chemistry
Testing gravity through the distortion of time
Sep 20, 2024 in Maths, Physics & Chemistry | 3 min read by Sveva CastelloStacking molecular chips in multiple dimensions
Aug 30, 2024 in Maths, Physics & Chemistry | 3 min read by Lucía Gallego , Romain Jamagne , Michel RickhausReversible Anticoagulants: Inspired by Nature, Designed for Safety
Jun 12, 2024 in Maths, Physics & Chemistry | 4 min read by Millicent Dockerill , Nicolas WinssingerDistance-preserving moves always keep a point fixed
May 18, 2024 in Maths, Physics & Chemistry | 4 min read by Shaula FiorelliA resonance triggers chemical reactions between the coldest molecules
Apr 5, 2024 in Maths, Physics & Chemistry | 3 min read by Juliana Park , Wonyl ChoiEditor's picks
Trending now
Popular topics


