 Health & Physiology
Health & Physiology
A new promising therapy against Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis, also known as Lou Gehrig's disease, is an uncurable and debilitating disease. The use of therapeutical stem cells represents an innovative approach to contrast the neurodegeneration causing the disease.
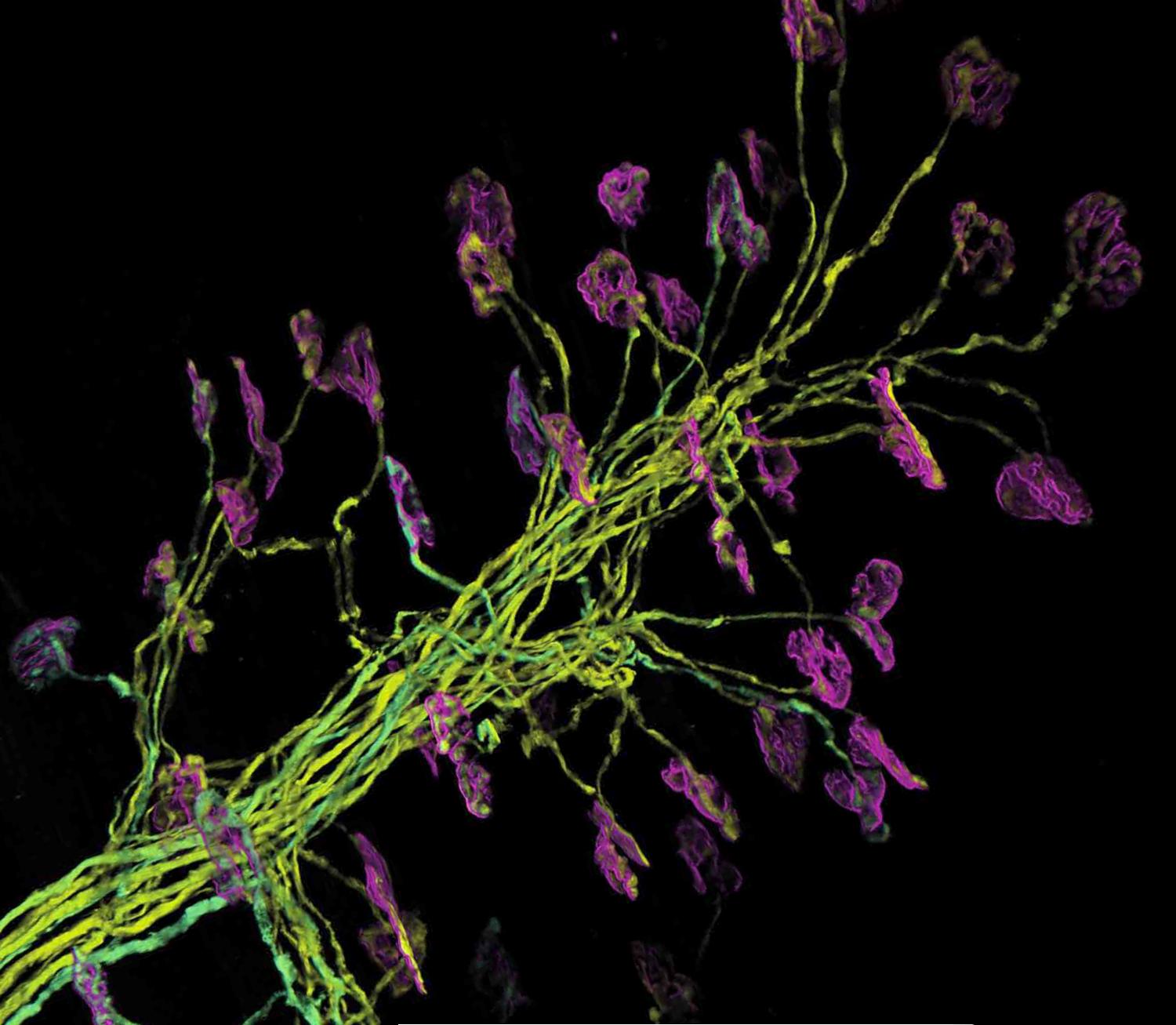
Amyotrophic lateral sclerosis (ALS) is an incurable disease, causing the death of the "motor" neurons that allow us to control our movement. ALS causes muscle atrophy, paralysis and death due to respiratory failure within two and five years. With a worldwide incidence of 2 cases per 100'000, ALS is mostly sporadic but in about 10% of the cases the members of a family can develop the same disease because of either genetic reasons or environmental causes.
ALS degenerates components of the nervous system by stimulating them in excess. To date, only a drug that dampens down that excessive stimulation, riluzole, is known to modestly increase patient survival.
Stem cell therapy is now standing out as a potential treatment for ALS. A stem cell therapy relies on the transplant of "unspecialized" stem cells that can turn into specific cell types - during a process called "differentiation" - able to restore the diseased tissue of the patient.
Nevertheless, there is a set of technical and ethical limits regarding the origin, isolation methods and safety of such stem cells which must be considered and overcome. The best stem cells for this kind of therapy emerge from human embryos or fetuses, but taking them from these sources represents a complex topic of ethical debate.
Stem cells originating from the nervous tissue of human fetuses that had an early death, called human neural stem cells (hNSCs), exhibit an important therapeutic potential when implanted in rodents with neurological diseases.
Interestingly, in rodents as well as non-human primates affected by a neurodegenerative disease, autoimmune encephalomyelitis, the implanted neural stem cells improved the condition of the sick animals. These cells integrated into the diseased tissue and turned into cells that produced neuroprotective molecules. Such neuroprotective molecules support the survival, growth and differentiation of the neurons. Regarding ALS, the implantation of hNSCs next to the dying motor neurons should stimulate them and other surrounding cells, called glial cells, which support and protect the neurons.
Such an injection of "therapeutic stem cells" in patients is possible today, since in 2011 a technique for the delivery of donor stem cells in proximity of the motor neurons in the spinal cord was developed.
Encouraging results from an initial clinical trial for ALS, conducted by Letizia Mazzini et al., were published in 2015: hNSCs isolated from spontaneously aborted fetuses were transplanted into the spinal cord of ALS patients, testing for the safety of both cells and neurosurgery. Six sporadic ALS patients with a median age of 46 years received three or six microinjections of stem cells into the spinal cord. Neither adverse events or post-procedural complications were observed in the patients because of the treatment. They were clinically monitored after the hNSCs transplantation, firstly assessing the lack of acceleration of progression of the disease because of the treatment. Interestingly, a transitory improvement in the ability to walk was observed for two patients and it lasted three months while another patient showed an improvement in the strength of the tibia that lasted seven months; the remaining three patients died but due to the natural course of the disease. Even if we are dealing with slight clinical improvements, they can be considered encouraging.
The main result of the work of Letizia Mazzini et al. is that the intra-spinal cell injection of hNSCs in humans is a safe procedure and that such cell therapy lacks of toxic side effects. Another fundamental characteristic of this clinical trial is the fact that it is free from any ethical concerns, since the source of hNSCs is represented by fetuses that underwent spontaneous in utero death.
The cell therapy described here gives hope to ALS patients. Further efforts are in place in order to broaden the clinical trials described and we hope that they will definitely demonstrate the effectiveness of the stem cell therapy approach in the treatment of ALS, as well as other neurological disorders, in a short time.
Original Article:
Mazzini L, Gelati M, Profico D, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli M, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, Zalfa C, Binda E, Visioli A, Trombetta D, Novelli A, Torres B, Bernardini L, Carriero A, Prandi P, Servo S, Cerino A, Cima V, Gaiani A, Nasuelli N, Massara M, Glass J, Sorarù G, Boulis N, Vescovi A. Human neural stem cell transplantation in ALS: initial results from a phase I trial. Journal of Translational Medicine. 2015;13(1):17. doi:10.1186/s12967-014-0371-2Edited by:
Dr. Carlos Javier Rivera-Rivera , Managing Editor
We thought you might like
How scientists light up their tobacco
Nov 9, 2020 in Plant Biology | 3.5 min read by Louisa González SomermeyerThe snake with the skin of a rhino…that eats babies!
Sep 21, 2018 in Evolution & Behaviour | 3.5 min read by Dawei Han , Bruce A. YoungObesity: The heavyweight of cancer
Apr 12, 2018 in Health & Physiology | 4 min read by Daniela Quail , Oakley Olson , Johanna JoyceSize does not matter: direct estimations of mutation rates in baleen whales
Jan 29, 2025 in Evolution & Behaviour | 4 min read by Marcos Suárez-MenéndezMore from Health & Physiology
Tobacco smoking and other exposures shut off cancer-fighting genes
Aug 31, 2024 in Health & Physiology | 3 min read by Jüri Reimand , Nina AdlerA hidden clock that times cytoplasmic divisions
Aug 30, 2024 in Health & Physiology | 3 min read by Cindy OwWhen two kinases go for a dance
Aug 2, 2024 in Health & Physiology | 4 min read by Ioannis Galdadas , Francesco Luigi Gervasio , Pauline JuyouxAwakening the thymus to cure SARS-CoV-2 infection: a matter of genes
Jul 27, 2024 in Health & Physiology | 3.5 min read by Stefano Marullo , Cheynier RemiKeeping the balance: How epigenetics monitors cancer genes
May 13, 2024 in Health & Physiology | 4 min read by Zach Gray , Madison Honer , Johnathan WhetstineEditor's picks
Trending now
Popular topics


