 Maths, Physics & Chemistry
Maths, Physics & Chemistry
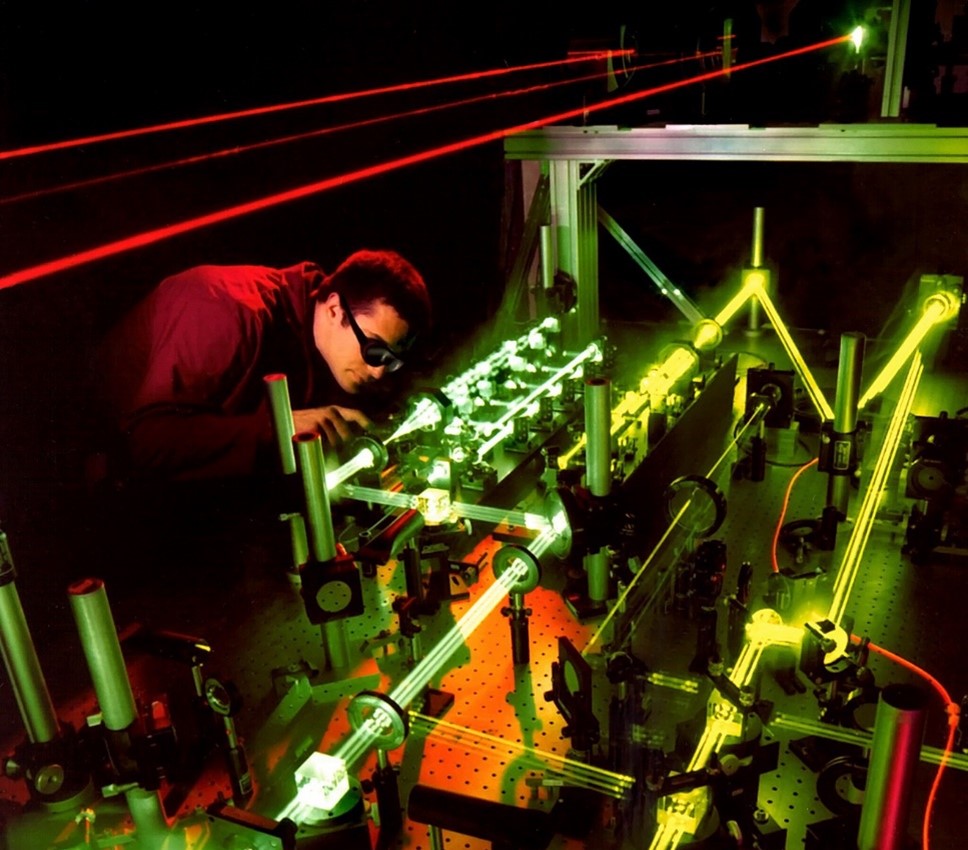
A laser proton accelerator targeting tumors in mice
High-power short-pulse lasers can generate intense but fluctuating bunches of energetic protons. We demonstrate a new level of stability for laser-driven proton sources that allowed us to successfully perform the world-wide first tumor irradiation study in mice with this novel radiation source.
Views 2896
Reading time 4 min
published on Jun 16, 2023
After developing the first laser in 1960, Theodore Maiman dubbed it “a solution looking for a problem”. Now, lasers are among the most important problem solvers in your everyday life: Your glasses are bothering you? Lasers! You want to get rid of that tattoo spelling your ex’s name? Lasers! Your cat needs exercise? Lasers!
For us researchers, the laser has become one of the niftiest gadgets in our toolbox. Under specific conditions, laser light can even accelerate charged particles. Particle accelerators are of great importance for science, industry, and society, with applications ranging from structural analysis to cancer therapy.
Here we focus on the acceleration of protons by means of laser light. Every atom consists of a positively charged nucleus surrounded by negatively charged electrons. A proton is the nucleus of the hydrogen atom. Laser-based proton acceleration relies on extremely intense bursts of light produced via a method called “chirped pulse amplification”. The development of this amplification technique was awarded with the Nobel Prize in Physics in 2018.
Intense laser pulses can strip the electrons from nuclei, turning the targeted material into what is called a plasma. When such a laser pulse is tightly focused onto a thin foil, the laser pushes the electrons outward while the sluggish nuclei stay in place. While the laser continues pushing the electrons, the nuclei start to move due to the strong attracting force of the electrons – the nuclei are accelerated and ejected as a dense bunch. The acceleration process is most efficient for the lightest nuclei, i.e., protons. These originate either from the foil itself, if it contains hydrogen (e.g., plastic), or from a contaminant layer (e.g., water) on the foil surface.
Owing to their short acceleration length and unique beam properties, laser-driven proton accelerators could complement conventional accelerators like cyclotrons for many applications. However, laser-driven proton sources have hardly been exploited so far because of the chaotic nature of the plasma acceleration process, which translates into fluctuations in proton energy and number.
In our new study, we demonstrated an unprecedented level of stability for laser-driven proton sources, qualifying them for irradiation of even the most delicate samples. Physicists, laser engineers, and radiobiologists pulled together to perform a first-of-its-kind study: The targeted irradiation of tumors in living animals with laser-accelerated protons. The success of the experiment rested on five strong pillars.
First, we developed a dedicated animal model: Human cancer cells were injected into the ears of mice to form small tumors that can easily be penetrated by protons of moderate energy. The tumors must be irradiated homogeneously with a prescribed radiation dose to delay tumor growth. We recorded the tumor growth for up to 120 days post irradiation. The mice were divided in different treatment and control groups.
Second, we optimized the laser plasma interaction to maximize the proton source stability: We found that the exact “temporal shape” of the laser pulse (its intensity distribution over time) is key to accelerate protons to consistently high energies and numbers. We improved and monitored the proton acceleration performance for two years before attempting the mouse irradiation.
Third, we set up a system to guide the beam and select the “right” protons for dose delivery: We used pulsed electromagnets specifically developed to match the pulsed proton source. On its way to the tumor, the proton bunch passes apertures and scatterers that fine-tune the bunch properties for homogeneous dose delivery.
Fourth, we designed proton detectors and dosimeters tailored to our setup: A suite of online and offline diagnostics allowed us to monitor every single proton bunch delivered to the tumors and precisely determine the applied dose.
Fifth, we prepared man and machine: Over months, our interdisciplinary team practiced the irradiation workflow. Rigorous daily quality assurance and laser maintenance were conducted.
In the end, we performed the dose-controlled tumor irradiation with our laser-driven proton source. In total, 61 mice were evaluated for radiation-induced tumor growth delay, including mice irradiated at a clinical proton accelerator (a cyclotron not a laser). We showed that our laser-accelerated protons slowed down tumor growth as much as the clinically-used protons. This proves that laser-driven proton sources can now contribute to complex biomedical research.
In the future, we plan to explore radiobiological phenomena that are not yet fully understood, e.g., the so-called FLASH effect. During a FLASH irradiation, the radiation dose is applied very rapidly to a tumor. The tumor is damaged to the same extent as with “slow” dose application, but healthy tissue is spared. In clinical practice, FLASH could help radiotherapy patients in their fight against cancer, ensuring tumor control and low side effects simultaneously. The intense and temporally short proton bunches of laser accelerators allow for the most rapid dose application currently achievable, making them especially suited to identify underlying mechanisms and quantify the effectiveness of FLASH.
Original Article:
Kroll, F., Brack, F.-E., Bernert, C., Bock, S., Bodenstein, E., Brüchner, K., Cowan, T. E., Gaus, L., Gebhardt, R., Helbig, U., Karsch, L., Kluge, T., Kraft, S., Krause, M., Lessmann, E., Masood, U., Meister, S., Metzkes-Ng, J., Nossula, A., … Beyreuther, E. (2022). Tumour irradiation in mice with a laser-accelerated proton beam. Nature Physics, 18(3), 316–322. https://doi.org/10.1038/s41567-022-01520-3
Edited by:
Zoé Valbret , Senior Scientific Editor
We thought you might like
More from Maths, Physics & Chemistry
Testing gravity through the distortion of time
Sep 20, 2024 in Maths, Physics & Chemistry | 3 min read by Sveva CastelloStacking molecular chips in multiple dimensions
Aug 30, 2024 in Maths, Physics & Chemistry | 3 min read by Lucía Gallego , Romain Jamagne , Michel RickhausReversible Anticoagulants: Inspired by Nature, Designed for Safety
Jun 12, 2024 in Maths, Physics & Chemistry | 4 min read by Millicent Dockerill , Nicolas WinssingerDistance-preserving moves always keep a point fixed
May 18, 2024 in Maths, Physics & Chemistry | 4 min read by Shaula FiorelliA resonance triggers chemical reactions between the coldest molecules
Apr 5, 2024 in Maths, Physics & Chemistry | 3 min read by Juliana Park , Wonyl ChoiEditor's picks
The lifetime of memories
by
Thomas Stefanelli
,
Pablo Mendez
A Weekend Camping is Just What the Doctor Ordered
by
Hannah Kent Ritchie
,
Ellen R. Stothard
,
Kenneth P. Wright
Trending now
How accurate is our memory?
by
Nicholas B. Diamond
What were the ice age ‘stilt-legged’ horses of North America?
by
Peter D. Heintzman
High performance silks deployed by web building wolf spiders
by
Sean J. Blamires
,
Mariángeles Lacava
,
Luis F. García
Unveiling the secrets of ancient Egyptian ink
by
Marine Cotte
,
Thomas Christiansen
,
Sine Larsen
How wombats poop cubes
by
Zoé Valbret
Popular topics


