 Maths, Physics & Chemistry
Maths, Physics & Chemistry
Tiny molecular probes reveal invisible forces inside cells
Tiny fluorescent molecules, inspired by the color-changing lobster pigment, have been developed to visualize and measure the tension of various cellular membranes. It is the first time scientists can observe the consequences of an invisible force acting in real-time inside cells.
To function and survive, all cells need to sense and respond to invisible physical forces. Being able to detect and measure these forces is thus key to our understanding of life. Still, it remains one of the most complex problems facing current Science.
In particular, biologists have wished to visualize the tension of cellular membranes for decades. Tension - the force developed when membranes are stretched - is indeed involved in regulating many important biological processes, including cell division, cell migration, or cell signaling. By better understanding how plasma membrane tension is linked to these normal processes, scientists might be able to develop potential treatments when their regulation is lost, causing diseases like cancer.
However, they have so far lacked a convenient and reliable tool to image and measure this invisible force.
Work carried out by biologists and chemists within a network of research excellence, NCCR Chemical Biology, has recently enabled the design, synthesis, evaluation, and commercialization of four fluorescent membrane tension probes, finally filling this important technical gap.
The design of the probes was initially inspired by a natural and striking phenomenon: how lobsters change color when they are cooked. The orange color of lobsters is due to a pigment present in their shell. When the animals are alive, the pigment is flattened by forces applied by surrounding proteins, which make it turn blue. Upon cooking, the high temperature causes these proteins to lose their defined structure. The pigment is then released from its proteic cage, changing its shape, and regaining its natural orange color.
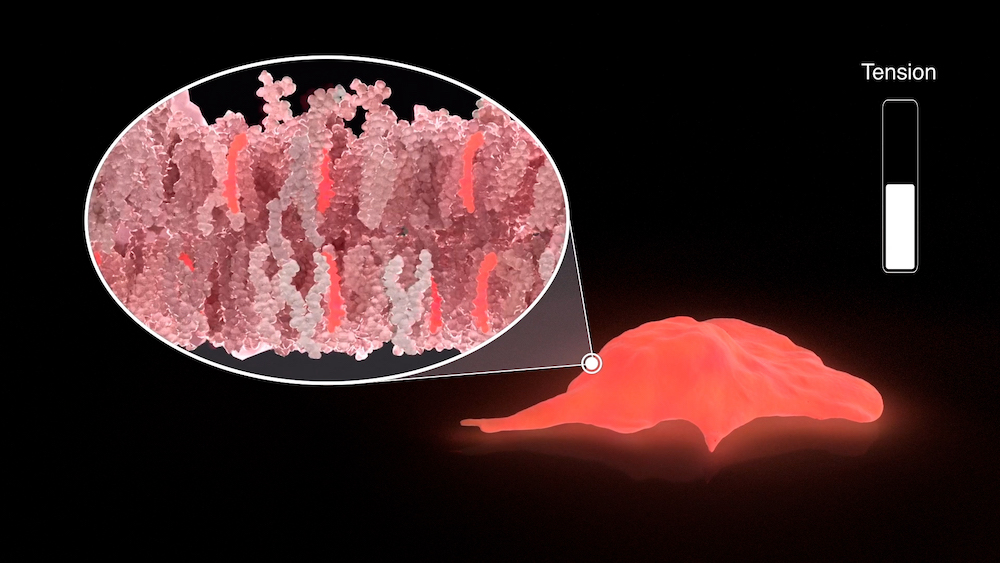
The chemists decided to adapt this concept to create tiny mechanosensitive molecules that would insert into biological membranes and behave similarly. It took them eight years to optimize the 15-step synthesis leading to the original probe “Flipper-TR” and precisely understand its mode of action.
“Flipper-TR” is a fluorescent molecule, meaning that it is able to transitively emit light of a specific color when ‘excited’ by a light of another precise color. Specifically, it contains two flat fin-like structures, connected by a chemical bond that lets them twist relative to each other depending on the strength of surrounding forces, such as tension. This structural change, in turn, modifies the fluorescence of the molecule, particularly its “fluorescence lifetime”. The fluorescence lifetime is the amount of time the molecule can emit light after excitation before it goes ‘off’ again. Scientists can measure this parameter using a technique called Fluorescence Lifetime Imaging Microscopy (FLIM) and have observed that it is directly proportional to membrane tension. Measuring the probe's lifetime by FLIM can thus be used to calculate the tension of the membrane in which it is inserted, which can then be visualized through arbitrary color-coding.
The original “Flipper-TR” probe specifically targets the external membrane of the cell. It has already been used as a research tool by other groups, contributing to showing that changes in membrane tension can influence intracellular biochemical signaling.
In parallel, the team of chemists has developed three other probes that function in the same way but target different cellular membranes: “Mito Flipper-TR” targets the membrane of the mitochondria, which are responsible for energy production; “ER Flipper-TR” targets the membrane of the endoplasmic reticulum, which is crucial for protein production; and “Lyso Flipper-TR” targets the membrane of the lysosomes, which can be compared to the recycling plants of the cell. Several other flipper probes have already been created and await larger distribution.
Indeed, what truly made the Flipper-TR saga a real success story was its instant reception within the global research community. Scientists from all over the world have been quick to send requests for the probes as soon as these were published. Researchers were indeed excited to finally be able to try their long-dreamt-of experiments and observe the consequences of an invisible force acting in real-time inside cells.
To meet the huge demand, the NCCR Chemical Biology had to organize and scale up the production of complex, top-quality material. Since 2018, hundreds of samples have been distributed worldwide through Spirochrome, and the Flipper-TR probes have already appeared in dozens of publications.
This is proof that this novel tool answers a long-time and crucial need from the research community, opening a brand-new door into the study of life sciences, and potentially, into the development of concrete treatments for many diseases.
View the graphical abstract of the article on Vimeo - Animation by Margot Riggi.
Original Article:
Assies, L. et al. Flipper Probes for the Community. CHIMIA International Journal for Chemistry 75, 1004-1011 (2021).Edited by:
Massimo Caine , Founder and Director
We thought you might like
Is prison the answer to preventing violence?
Dec 19, 2019 in Psychology | 3.5 min read by Anh P. NguyenA hidden clock that times cytoplasmic divisions
Aug 30, 2024 in Health & Physiology | 3 min read by Cindy OwDistractions and emotions: key factors in vehicle collisions
Jun 30, 2016 in Psychology | 3 min read by Jon HankeyFish identify themselves in mirrors and portraits
Oct 18, 2023 in Evolution & Behaviour | 3.5 min read by Masanori Kohda , Satoshi Awata , Shumpei SogawaMore from Maths, Physics & Chemistry
Testing gravity through the distortion of time
Sep 20, 2024 in Maths, Physics & Chemistry | 3 min read by Sveva CastelloStacking molecular chips in multiple dimensions
Aug 30, 2024 in Maths, Physics & Chemistry | 3 min read by Lucía Gallego , Romain Jamagne , Michel RickhausReversible Anticoagulants: Inspired by Nature, Designed for Safety
Jun 12, 2024 in Maths, Physics & Chemistry | 4 min read by Millicent Dockerill , Nicolas WinssingerDistance-preserving moves always keep a point fixed
May 18, 2024 in Maths, Physics & Chemistry | 4 min read by Shaula FiorelliA resonance triggers chemical reactions between the coldest molecules
Apr 5, 2024 in Maths, Physics & Chemistry | 3 min read by Juliana Park , Wonyl ChoiEditor's picks
Trending now
Popular topics


