 Health & Physiology
Health & Physiology
Vicious Circles – how changes in the shape of DNA can drive cancer
One of the most iconic images in biology is the 23 pairs of chromosomes in the human cell. It is central to the paradigm of inheritance. Our recent findings show that cancer breaks that rule. We have explained how changes in the shape of DNA, as it forms a circle, promotes cancer. This scenario makes cancer to evolve quickly and more resistant to treatments.
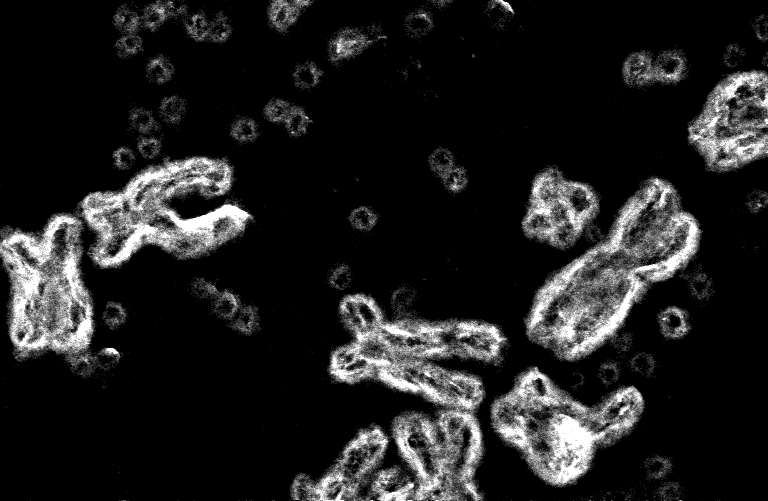
The diploid human genome contains 23 pairs of chromosomes whose DNA encodes genes for life activities, such as cell division. Cancer corrupts those genes, making growth-promoting genes more active (oncogenes) or growth-inhibiting genes (tumor suppressors) less active. One of the most common genetic alterations causing cancer is oncogene amplification. Instead of 2 copies, the tumor cell might have 10, 20, 30, or even more copies of an oncogene, leading to unrestrained growth.
The development of relatively low-cost, high-throughput sequencing, and the development of a human genome map have been critical tools for precision medicine, making it possible to ask what are the genetic alterations that have occurred in a person's tumor and trying to use this information to tailor individualized treatment. However, we discovered that many of the amplified oncogenes are not actually found where the maps indicate. Instead, they seemed to reside on extrachromosomal DNA particles.
Extrachromosomal DNA (ecDNA) was first described in cancer in 1965, but it was thought to be a rare event (1.4% of cancers). In 2014, we discovered that some cancers could evade treatment when the oncogene was present on extrachromosomal DNA (ecDNA). This evidence led us to revisit the question regarding the prevalence and significance of ecDNA in cancer. We built a pipeline to integrate sequencing with visual assessment of where specific pieces of DNA actually reside. In 2017, we showed that ecDNA is common, present in a quarter to a third of all cancers. Further, ecDNA enables high oncogenes copies and cell-to-cell variability in the oncogene numbers. Therefore, pulling the oncogene off the chromosomal as ecDNA allows tumors to evolve quickly and resist treatment.
Given the prevalence and importance of ecDNA in cancer, we began to wonder whether the shape of ecDNA could make cancers more aggressive. We explored the shape of ecDNA first by applying a new algorithm able to decipher the potential structure of the DNA starting from its sequence. We then complemented that analysis with a powerful new tool called optical mapping to read very long stretches of DNA. Integrating these tools demonstrated circular structures. However, seeing is believing. Therefore, we used powerful visualization techniques to peer into the structure of the nucleus, demonstrating that ecDNA is circular - unequivocally.
Chromosomal DNA serves as a template to direct transcription of messenger RNA (mRNA) to express genetic information. Likewise, ecDNA is also actively transcribing mRNA. But ecDNA is making a lot more of mRNA. Especially for the oncogenes on the ecDNA, the abundance of their transcripts usually ranks higher than 1% of all genes. We reasoned that the massively increased gene expression was due to their high copy number caused by ecDNA. For example, it is not difficult to find an oncogene that amplifies to more than 100 copies. Still, it is rare to see such a high copy number on chromosomal DNA.
We also sought to resolve the organization of ecDNA, to understand how it contributed to high levels of oncogene expression. On chromosomes, the DNA helix is wound around protein cores, which enables the DNA to compress and fit into the nucleus of the cell, and also controls which pieces of DNA are accessible to the transcriptional machinery. Accessible DNA can be transcribed, and inaccessible DNA cannot. Our team used newly developed tools to show that the double helix of DNA on ecDNA is wound around protein cores but in a much more accessible fashion. This organization contributes to the massive level of oncogene expression that will ultimately make the proteins to drive cancer growth.
Chromosomal DNA is organized into loops that bring regulatory DNA elements into proximity of coding genes to regulate their expression. This interaction is confined in a certain range. As the distance between two DNA elements increases, the interaction frequency decreases. Strikingly, we find that the circularity of ecDNA changes the paradigm. The circularization brings distal DNA element into proximity, thus enabling ultra-long-range interaction, creating a new regulatory circuit for gene expression.
Our findings suggest that where oncogene-encoding DNA locates and the shape of that DNA may be fundamental for some aggressive cancer. These discoveries suggest how changes in the shape and location of DNA in cancer contribute to treatment resistance. We hope that our research will provide new insight into this dreaded disease and yield new ways to think about cancer genetics so that we can collectively leverage this knowledge to develop more effective treatments for cancer patients.
Original Article:
Wu S, Turner K, Nguyen N et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. 2019;575(7784):699-703.Edited by:
Massimo Caine , Founder and Director
We thought you might like
GMOs are not a human invention: sweet potato is a naturally transgenic food crop
Jul 6, 2015 in Plant Biology | 3 min read by Tina KyndtA new code for a new life
May 26, 2016 in Maths, Physics & Chemistry | 3.5 min read by Jordan CostafrolazAmoebas trap bacteria using nets of DNA: the same mechanism as human immune cells
Jan 27, 2017 in Evolution & Behaviour | 3.5 min read by Lukáš NovákWhat happens to our genes in the twilight of death?
Feb 28, 2017 in Health & Physiology | 3.5 min read by Peter Noble , Alex PozhitkovMore from Health & Physiology
Tobacco smoking and other exposures shut off cancer-fighting genes
Aug 31, 2024 in Health & Physiology | 3 min read by Jüri Reimand , Nina AdlerA hidden clock that times cytoplasmic divisions
Aug 30, 2024 in Health & Physiology | 3 min read by Cindy OwWhen two kinases go for a dance
Aug 2, 2024 in Health & Physiology | 4 min read by Ioannis Galdadas , Francesco Luigi Gervasio , Pauline JuyouxAwakening the thymus to cure SARS-CoV-2 infection: a matter of genes
Jul 27, 2024 in Health & Physiology | 3.5 min read by Stefano Marullo , Cheynier RemiKeeping the balance: How epigenetics monitors cancer genes
May 13, 2024 in Health & Physiology | 4 min read by Zach Gray , Madison Honer , Johnathan WhetstineEditor's picks
Trending now
Popular topics


