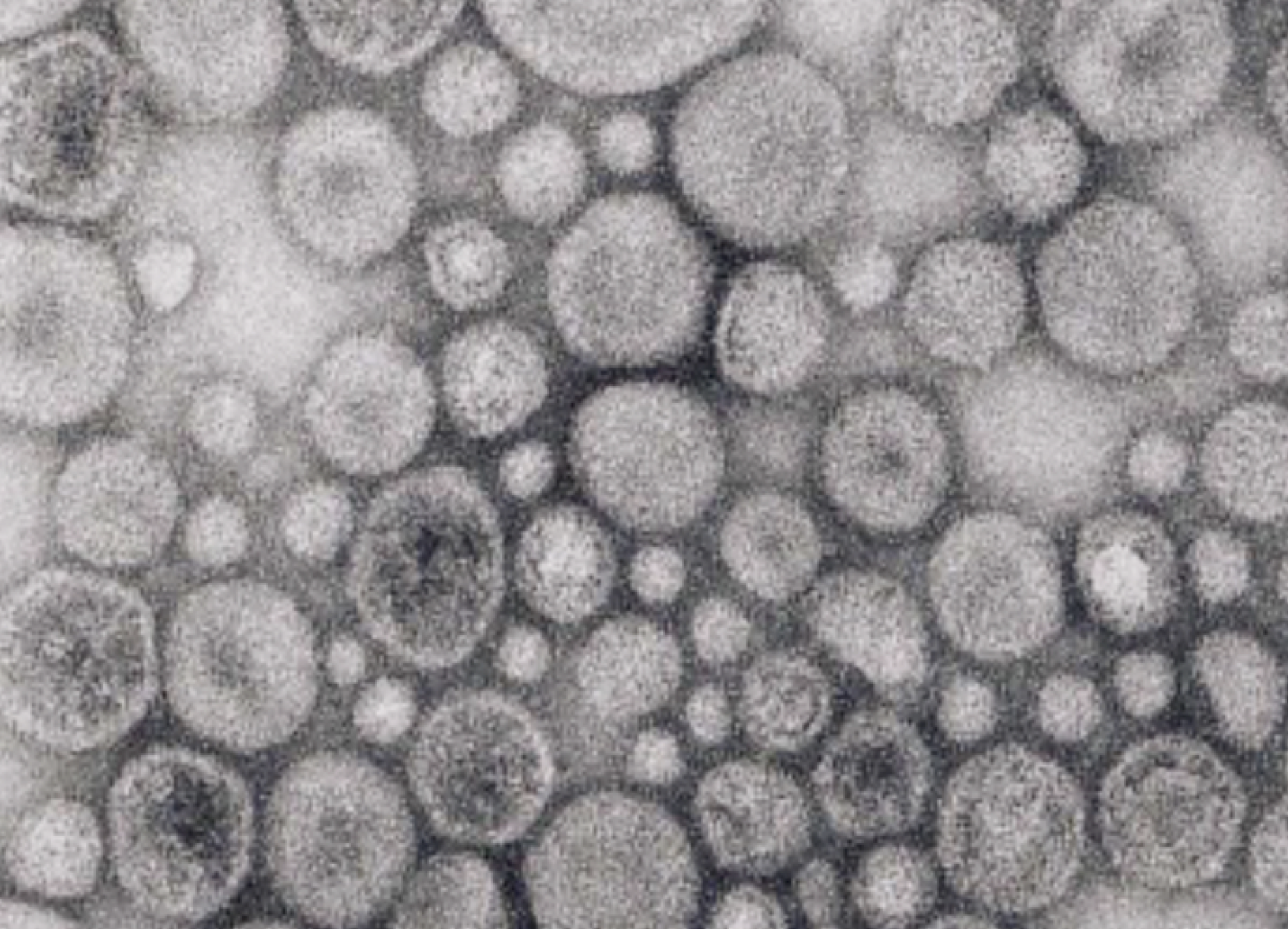
We investigated how the gut microbiota modulate anti-viral immunity in mouse models of infection. We showed that gut bacteria are vital for the presence of low level of type-I interferons, key mediators in anti-viral immunity. The gut bacteria deliver DNA in vesicles to host cells, priming anti-viral immunity.
The bodies of multicellular organisms, including humans, are covered with commensal microbes, also called the microbiota. The majority of these are bacteria located in the gastrointestinal tract. The gut microbiota maintain many physiological functions including the development and maturation of the immune system. The microbiota also act as a competitive barrier against invading pathogens at body surfaces. A lot of research investigates interactions of pathogenic microbes with host cells and how pathogenic infections may cause damage to their hosts. However, how the gut microbiota interact with and shape host immunity to pathogens, in particular viral infections, is only beginning to gain traction.
In our study, we investigated how gut commensal bacteria modulate anti-viral immunity. Key players in anti-viral immunity are type-I interferons (interferon-Is) that are expressed at high levels after infections and are involved in clearing viruses from the body. These interferon-Is are also expressed under non-infectious conditions, albeit at a low level. First, we investigated whether the gut microbiota have an impact on viral infection. Mice treated with an antibiotic cocktail via the drinking water, to suppress the gut microbiota, and then infected with herpes simplex virus type 1 became more susceptible to viral infection, compared to those not treated with antibiotics. This indicated that the gut microbiota are essential for innate resistance to viruses. Then we used reporter knock-in mice to monitor the expression of type-I interferons in real-time. These mice carry an additional “Firefly luciferase” gene meaning they emit light when interferon-I expression is induced. Thus, we could measure small changes of interferon-I levels in live mice at any time. We found that mice treated with antibiotics had reduced interferon-I levels, which correlated with diminished anti-viral immunity. This suggests that the gut microbiota increases interferon-I levels which drives anti-viral immunity.
The innate immune system is one of two branches of the vertebrate immune system that coordinates the initial response to invading microbes. The body’s cells are equipped with innate immune receptors for sensing microbes and many different innate immune sensors have been proposed to be involved in recognising microbes that drive the expression of interferon-Is. These sensors include receptors located on the cell surface that are involved in sensing microbes in the extracellular milieu. Other immune sensors are located inside the host cells where they sense intracellular pathogens such as viruses. Key among the intracellular receptors is a DNA receptor called cGAS.
We investigated which innate immune receptors are involved in recognizing the gut microbiota and inducing basal interferon-I expression. For this, we used interferon-I reporter mice deficient in different innate immune pathways. We found that cGAS was essential for innate immune recognition of the gut microbiota and the associated interferon-I response. This was surprising because the gut microbiota consist of extracellular bacteria but was sensed by an intracellular receptor. Even more surprising was that, when exploring the mechanism of how these bacteria induce an interferon-I response, we found that no direct contact between the bacteria and the host cells was required to induce an interferon-I response.
Therefore, we wondered, if direct host-bacteria contact is not needed, how are extracellular bacteria able to deliver DNA into host cells to activate cGAS signaling? Bacteria are known to produce membrane vesicles. We found that such vesicles contain bacterial DNA encased within their lumen. This led us to consider whether these vesicles delivered DNA from gut bacteria to distant host cells. Indeed, we purified bacterial membrane vesicles and we observed that they do induce interferon-I signaling in a cGAS-dependent manner and that this leads to increased anti-viral immunity in cell culture systems and in live mice. Moreover, we were able to isolate membrane vesicles that contain bacterial DNA from the mouse colon and blood. Thus, by shedding DNA-containing vesicles into the blood circulatory system, gut bacteria prime the interferon-I system via cGAS to protect distal organs from viral infections.
These novel insights into the communication between intestinal microbiota and innate immunity reveal the importance of the microbiota in maintaining the innate immune system in a constant state of alert against viruses. Moreover, our study reinforces that uncontrolled use or abuse of antibiotics by self-medicating patients might be an underestimated risk since the disruption of the gut microbiota by antibiotics may impair the body's ability to combat viral infections.
Original Article:
Erttmann, S. F., Swacha, P., Aung, K. M., Brindefalk, B., Jiang, H., Härtlova, A., Uhlin, B. E., Wai, S. N., & Gekara, N. O. (2022). The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity, 55(5), 847-861.e10. https://doi.org/10.1016/j.immuni.2022.04.006
 Health & Physiology
Health & Physiology