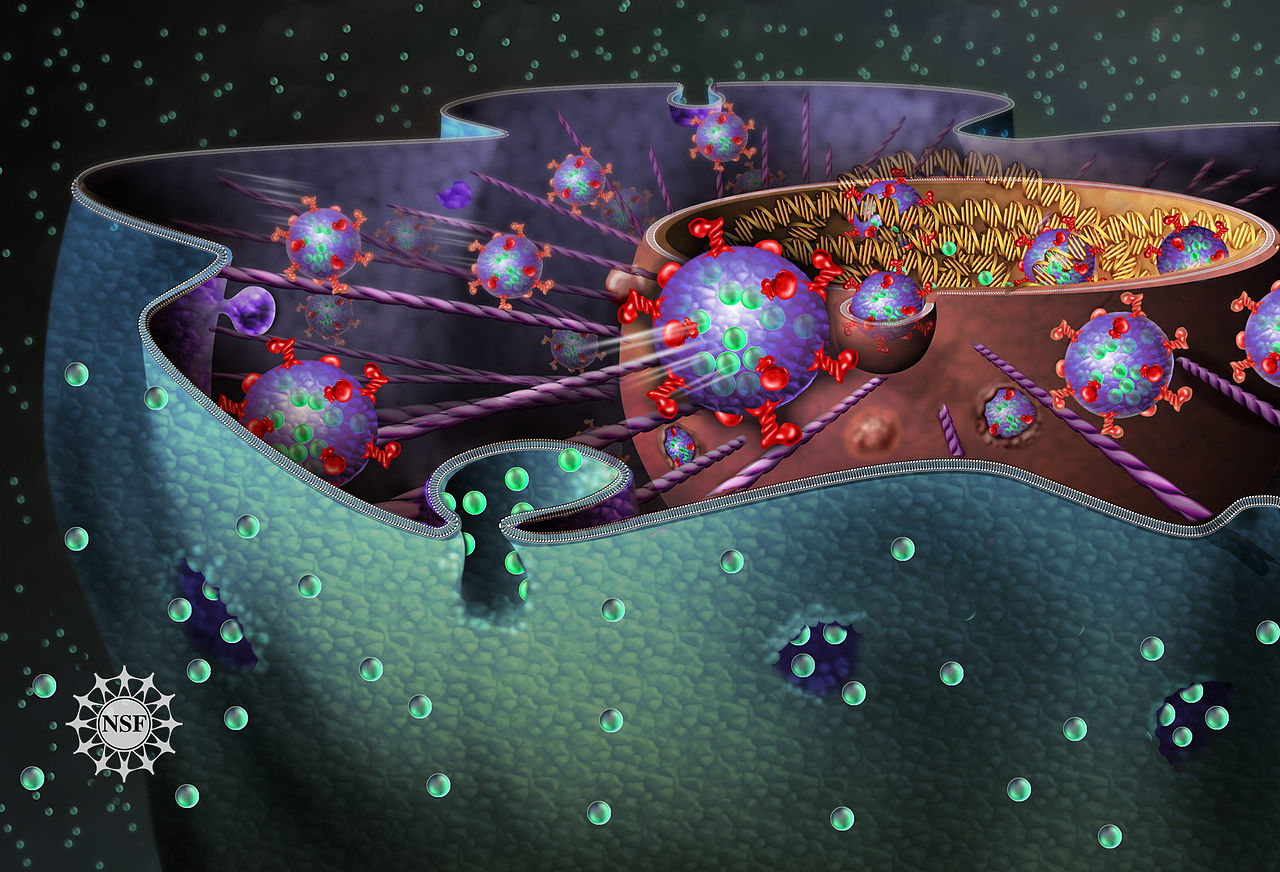
Retroviruses like HIV-1 enter the nucleus of immune cells to infect people, but it has been unclear how a relatively large virus can pass through the nuclear envelope or its small nuclear pores. We found a previously unknown pathway of entry, in which a virus-containing package causes invaginations to form in the nuclear envelope. Our work reveals new drug targets for limiting viral infections.
Views 1100
Reading time 4 min
published on Nov 17, 2023
Viruses need to be better understood. After all, the common cold deals misery to hundreds of millions of people every year, and the world stood still for almost a year waiting for a vaccine against SARS-CoV-2. Viruses are difficult to treat because they are simple, consisting of genetic material (RNA or DNA) and a protein coat called a capsid. They can reproduce only if they enter host cells and take over the manufacturing systems inside.
Research on viral infection of host cells has largely focused on cell entry, resulting in anti-viral drugs that interfere with viral entry into the cell. This makes sense for most RNA viruses, which enter the cell and reproduce outside the nucleus. However, those with DNA and some with RNA, like human immunodeficiency virus-1 (HIV-1), must be in the nucleus of the cell to establish an infection, so there is an additional place to block entry as they pass through the nuclear membranes, also known as nuclear envelope. Up to this point, it hasn’t been well understood how a virus that is inside the cell gets into the nucleus. The nuclear envelope has some holes, called nuclear pores, but viruses are generally much too large to passively make their way through.
We used human cells cultured in the lab, infected with a modified HIV-1 virus that could be handled more safely than the original. Some experiments were done using T cells, a type of immune cell which is the main target of HIV-1 in patients, so we could be sure our findings were relevant to human infections. Certain proteins were fluorescently labeled so we could track the infection process using a super-resolution microscope. In previous studies, we had identified proteins that were involved in transporting non-virus cargo across the nuclear envelope. We hypothesized that the process used to transport non-virus cargo was also being used by viruses to sneak into the nucleus.
We found that HIV-1 gains access to the cell nucleus through the interaction of three proteins, which form the VOR complex. First, the virus enters the cell by endocytosis, which refers to entering a cell by being engulfed by the cell membrane to form a membrane-covered package called an early endosome. As it traverses the cell, it matures into a late endosome. Late endosomes have the first member of the VOR complex, a protein called Rab7, embedded in the membrane which covers them. The second member of the VOR complex is another membrane-embedded protein called VAP-A, which is embedded in the nuclear envelope. The third protein, called ORP3, interacts with Rab7 and VAP-A to form the VOR complex.
We observed HIV-1 entering cells by endocytosis. The virus-containing endosomes matured to late endosomes and interacted with the nuclear envelope using the VOR complex to create invaginations. The virus-containing endosomes moved into the space within the invaginations, and the contents were transferred through the nuclear envelope. To understand how the VOR complex might be involved in the process, we interfered with VAP-A or ORP3 in a genetically targeted way, and we also used a molecule called PRR851, which inhibits formation of the VOR complex and has potential as a new drug. Whenever we interfered with formation of the VOR complex, the number of nuclear envelope invaginations was severely reduced, and there was no evidence that HIV-1 could enter the nucleus and start replicating.
This is a new pathway, so there are many unanswered questions. For example, we do not know how many other proteins are involved in the process, why the endosomes with virus inside are not degraded by the cell, how the virus may come apart and pass through the nuclear envelope, and how many other viruses use this pathway. Armed with new information about how viruses can enter the cell nucleus, we hope this discovery leads to new approaches for addressing viral diseases.
An interesting side note is that the first paper about this pathway came from a few members of our author team who are cancer researchers, not virologists. They found that tumor cells talk to other non-tumor cells in the body by sending signals that enter by endocytosis. The tumor signal endosomes access the nucleus using the same pathway that HIV-1 used in the current study. Cells that received such signals changed in ways that would allow metastasis (spread of cancer to other parts of the body) to occur. There is still much to learn, but it appears this pathway and its inhibitors may be relevant to more than just HIV-1.
Original Article:
Santos, M.F., Rappa, G., Karbanová, J., Diana, P., Cirrincione, G., Carbone, D., Manna, D., Aalam, F., Wang, D., Vanier, C., Corbeil, D., Lorico, A. HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells. Nat Commun 14, 4588 (2023).
 Health & Physiology
Health & Physiology